A Quality Undertaking
Connecting EQA and industry to ensure quality diagnostic testing
Beth Sheppard, Colin Tristram, John Garratt, Espen Walker, Jacqueline Hall |
At a Glance
- External quality assessment (EQA) ensures that diagnostic tests are reliable, accurate and translatable between laboratories
- Each laboratory’s selection of tests and procedures is different, and not all participate in similar EQA schemes, which makes quality assurance challenging
- Organizations like the International Quality Network for Pathology can bring together companies, EQA providers, and end users for the education of all
- With education comes greater understanding and the ability to shape future product development to better meet pathologists’ needs
Independent organizations around the world perform external quality assessment (EQA), or proficiency testing, to ensure that diagnostic laboratories produce valid and accurate test results. The need is clear – when using immunohistochemistry (IHC)-based diagnostics, for instance, it is critical to know (and be able to prove) that the staining results are accurate so that pathologists, physicians and other healthcare professionals can reliably use the tests to guide patient treatment.
Ensuring accuracy and precision in the EQA process is easier said than done. Different laboratories use different tests and procedures, resulting in variation across the field, but EQA schemes need to demonstrate accurate testing regardless of procedural variations within a laboratory or group. How is this accomplished?
- EQA schemes may provide universal standardized cell lines or tissue samples, and may involve centralized review of staining.
- EQA providers provide feedback and suggestions for improvement, for example on the reporting of test results.
- As EQA is a continuous process it provides ongoing learning opportunities and often the chance to participate in workshops setting best practice standards and guidelines.
The importance of EQA participation is underscored by the fact that accrediting agencies, such as the Internal Standardization Organization (ISO) and the College of American Pathologists (CAP) require it. In fact, in some jurisdictions, diagnostic laboratories must adhere to the standards established by their regional EQA agencies to receive reimbursement for any completed test. In the United States, for instance, laboratories who have difficulty passing EQA assessments are reported to the Centers for Medicare and Medicaid Services (CMS); in the United Kingdom, EQA participation is mandatory and laboratories that have persistently poor performance are reported to a committee who decides the appropriate course of action and investigation. In some jurisdictions, laboratories cannot even gain accreditation without EQA – in Canada, for example, testing requirements for crizotinib therapy mandate participation in an EQA scheme for predictive biomarkers associated with targeted therapies. High-quality, harmonized EQA activity is so vital that the World Health Organization (WHO) also recognizes its importance; in August of 2016, it released a document (1) entitled, “WHO manual for organizing a national external quality assessment program for health laboratories and other testing sites.” The guideline contains principles for the governance and operation of EQA schemes designed to assist in considering all the necessary factors, establishing a program, and reaching best practice standards at every level of healthcare.
Industry’s helping hand
Standardization isn’t easy. There are myriad factors to consider – establishing processes, streamlining operations, choosing references – and each one can have a knock-on impact on quality assurance and improvement.
Recognizing the inherent difficulties in standardization, EQA providers came together to form the International Quality Network for Pathology (2), a not-for-profit group that aims to encourage global harmonization by seeking areas for potential cooperation between EQAs providers, laboratories, diagnostic companies and patients (see Figure 1).
IQN Path’s goals are multiple:
- To establish benchmarks and best practice for EQA.
- To promote the use of EQA and to provide education on quality for diagnostic testing (including laboratory outreach, personnel training, and educational workshops).
- To create a communication platform on EQA, allowing stakeholders to participate in working groups to exchange expertise on new biomarker tests, upcoming assessments, and the challenges they encounter.
- Professionally recognize best practice in EQA via the EQA Provider Scheme standards.
- Develop internationally recognized standards, controls, tools and data resources (questionnaires, websites, templates and more) to support EQA providers and laboratories.
- Facilitate data sharing by pooling anonymized EQA performance data information from multiple providers to identify areas of suboptimal testing and provide data-driven suggestions for quality improvements.
A recent example of a successful IQN Path project was the launch of a survey and pilot EQA on cell-free DNA (cfDNA) testing for lung and colorectal cancer. Testing cfDNA mutations to manage patients’ treatment is a novel technology that has now entered the clinic. With any new form of testing used for patient management, we must ensure appropriately designed EQA. In this case, four providers came together under the IQN Path umbrella to establish best practice standards and design a pilot cfDNA EQA and consensus scoring system. A survey of 164 laboratories indicated that 17 percent already used cfDNA testing to inform patient treatment, and many more are preparing to use it. The pilot EQA organized under IQN Path offered a chance for the providers to discuss and design appropriate reference materials and scoring systems and harmonize their use. Any organization can access this type of support; the IQN-Path application process gives them the opportunity to request funding and support for projects that benefit the international pathology community at large, and allows the IQN Path Board to strategically support initiatives and to allocate resources appropriately.
Education in action
Cancer immunotherapy – and the many companion diagnostics that accompany it – have occupied a prominent place in recent scientific news. PD-L1 is normally associated with immune homeostasis, but overexpressed in many cancers, binding to surface markers on cytotoxic T lymphocytes and preventing the natural anti-tumor immune response. Tumors that overexpress PD-L1 are susceptible to treatment with immune checkpoint inhibitors – so knowledge of a cancer’s PD-L1 status is key to selecting the most effective treatment.
Currently, there are four different FDA-approved clinical tests for PD-L1. In addition, there are several antibody clones employed in laboratory-developed tests (LDTs) with commercially available cell line controls. The adoption of LDTs is driven by cost – they are usually far cheaper than commercial assays – but those savings are often offset by the cost of mandatory validation to ensure accuracy.
IQN Path is now launching a new project – a digital, educational self-assessment on PD-L1 readout. The online portal will feature images of PD-L1-stained cases with associated H&E slides, covering the four different FDA-approved kits, and provide a learning opportunity for pathologists to test their skills and receive feedback by comparing their results to the gold-standard scoring for each sample (established by an expert pathologist committee). Anyone can participate by creating a login via an EQA provider of their choice; there is no requirement for previous engagement with that provider, although we recommend that laboratories performing PD-L1 testing also join an EQA scheme covering the analytical phase of testing that includes the provision of physical samples for staining.
A forum for discussion
In February 2017, IQN Path staged an educational forum on FDA-approved PD-L1 assays, bringing EQA members together with industry professionals to share expertise. The meeting had four main goals:
- To acknowledge the complexity of the current diagnostic landscape of PD-L1;
- To inform EQA members about the available assays;
- To provide EQA members with training from industry experts on the specifics of those assays and their assessment;
- To give EQA providers the opportunity to ask questions, raise issues and provide feedback on recent and upcoming EQA rounds involving PD-L1 testing.
The meeting included EQA delegates from Europe, China and the United States who represented organizations across all fields of diagnostics. Topics included case reviews and assays, as well as good practice and potential pitfalls related to PD-L1 testing. The open forums were particularly useful, facilitating vigorous and productive discussion between EQA representatives and the drug and medical device delegates. As with all IQN Path meetings, this forum included diagnosticians as well as industry representatives, making it fertile ground for innovative thought and discussion.
Delegates left the meeting with two key messages: first, that industry and EQA agencies must work closely together to understand the practical aspects of assay implementation in clinical laboratories; and second, that training and education in assay interpretation are critical to properly assess the assay’s performance.
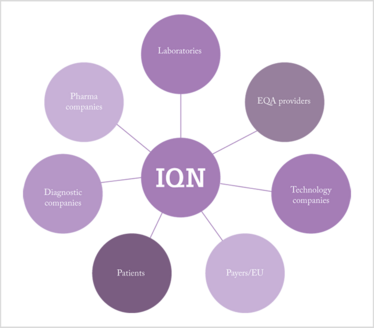
Figure 1. The stakeholders involved in biomarker testing quality, one area where EQA is rapidly evolving.
Did it work?
Feedback from delegates indicated that the meeting was extremely useful, with an overall mean satisfaction score of 4.9 on a 5.0 scale – a clear indication of the demand for such training among EQA providers. The open forums in particular yielded vigorous and productive discussions between EQA representatives and the drug and medical device delegates. As a result, the providers ended up with a more nuanced understanding of the complexities involved in developing a fully validated assay, and learned about the staining patterns and scoring algorithms directly from the pathologists who had developed the assays.
It’s not only the quality assurance professionals who benefit from such collaborations, though. The industry partners who co-hosted the event found themselves better able to understand the needs of EQA professionals, and gained a first-hand perspective of the quality assessment methods used by different organizations. In the future, this type of insight should allow industry professionals to assist EQA providers in setting up testing schemes – providing additional benefit for those providers, and for the laboratories who ultimately make use of external quality checks.
Of course, this is only one meeting – but it serves as a model to help open lines of communication between assay development companies and the pathologists who are implementing those assays in their laboratories. By sharing their goals and ideas, participants can help tailor future product development to better meet their laboratories’ – and their patients’ – needs.
The future of EQA
We need to investigate new ways of supporting EQA. Digital pathology and online assessments like the PD-L1 readout self-assessment project are good examples – although these digital tools should complement assessments of the analytical phase. Another is the identification of common requirements for reference samples between EQA providers, which we need to promote harmonization and develop shared best practices.
EQA in diagnostic laboratories is critical to safeguarding the health and safety of patients through best practices. Our educational forum was the first formal collaboration between IQN Path, a medical device company, and the EQA organizations representing regions across the globe. Organizations like IQN Path can link drug and medical device manufacturers, EQA scheme facilitators, end users and other interested parties together in a neutral environment. By bringing together like-minded and committed contributors to safely and openly discuss concerns and solutions, they can help diagnostic laboratories to deliver accurate, high-quality results in a timely fashion.
- World Health Organization, “WHO manual for organizing a national external quality assessment programme for health laboratories and other testing sites” (2016). Available at: bit.ly/2eOTIBx. Accessed September 2, 2017.
- IQN Path, “International Quality Network for Pathology” (2016). Available at: www.iqnpath.org. Accessed September 2, 2017.
Beth Sheppard is Senior Director of Market Access at Ventana Medical Systems, Inc. (Roche Tissue Diagnostics), Tucson, USA.
Colin Tristram is Co-Founder and Director of HistoCyte Laboratories Ltd., Newcastle upon Tyne, United Kingdom.
John Garratt is Scheme Manager at Canadian Immunohistochemistry Quality Control (cIQc), Vancouver, Canada.
Espen Walker is Medical Affairs Director at Ventana Medical Systems, Inc. (Roche Tissue Diagnostics), Tucson, USA.
Jacqueline Hall is Executive Director of IQN Path, Luxembourg.