
In the rapidly evolving field of precision oncology, the implementation of next-generation sequencing (NGS) has become a game-changer for diagnostic laboratories worldwide. Recently, we had the opportunity to interview Professor Louise Burke, Professor of Pathology, University College Cork and lead Thoracic Pathologist, Cork University Hospital, which serves a population of approximately 1.4 million people. The hospital’s tertiary cancer facility, part of Ireland’s National Cancer Control Program, has successfully transitioned from single-gene oncology biomarker testing to the sophisticated realms of NGS. Here, we explore their journey, challenges, and significant improvements in their biomarker testing capabilities following NGS accreditation.
What prompted your laboratory to transition to NGS technology?
We were doing single gene tests, but we really needed to expand our expertise to be able to meet the clinical demands. Over the past five years, we’ve seen a five-fold increase in requests driven by advancements in precision oncology. It was essential for us to integrate NGS seamlessly into our workflow while ensuring compliance with ISO 15189 requirements.
What were some of the initial challenges you faced in implementing NGS?
Transitioning to NGS was certainly not without its hurdles. We were NGS naive without in-house bioinformatics expertise, which is crucial for the successful implementation of NGS. However, we were determined to advance our diagnostic capabilities despite these obstacles.
What were the key factors you considered when choosing the NGS technology?
We needed a system that would fit within our existing infrastructure and staffing resources. We wanted something that had minimal hands-on time. Ease of integration and simplicity were also critical, ensuring a smooth and efficient transition.
What improvements have you noticed since implementing NGS?
One of the most notable advancements is the dramatic reduction in turnaround time. With NGS in-house, we’ve achieved greater than fifty percent reduction in turnaround time within the first year, achieving turnaround times of seven days, and we’ve further improved on that. This efficiency has been maintained despite an increase in workload, enabling us to provide exceptional service to oncologists and patients alike.
NGS has empowered us to better manage tissue samples, particularly in cases of non-small cell lung cancer (NSCLC) where sample size and quality are critical. Additionally, the implementation of NGS has fostered an educational program within our laboratory and opened numerous multidisciplinary collaborations with clinical colleagues and academic institutions – enriching the professional development of all staff members involved.
Can you summarize your experience with NGS accreditation?
The pursuit of ISO 15189 accreditation was driven by our commitment to delivering the highest standards of quality and reliability in genomic testing. This accreditation ensures that our testing processes meet stringent international criteria, providing confidence to healthcare providers and patients.
Achieving accreditation was a rigorous yet rewarding process. We, of course, had experience from accrediting other methods in past, and the template is generally the same. One of the key elements is ensuring that we can demonstrate our performance metrics reliably and reproducibly. It requires meticulous documentation and validation of all our processes.
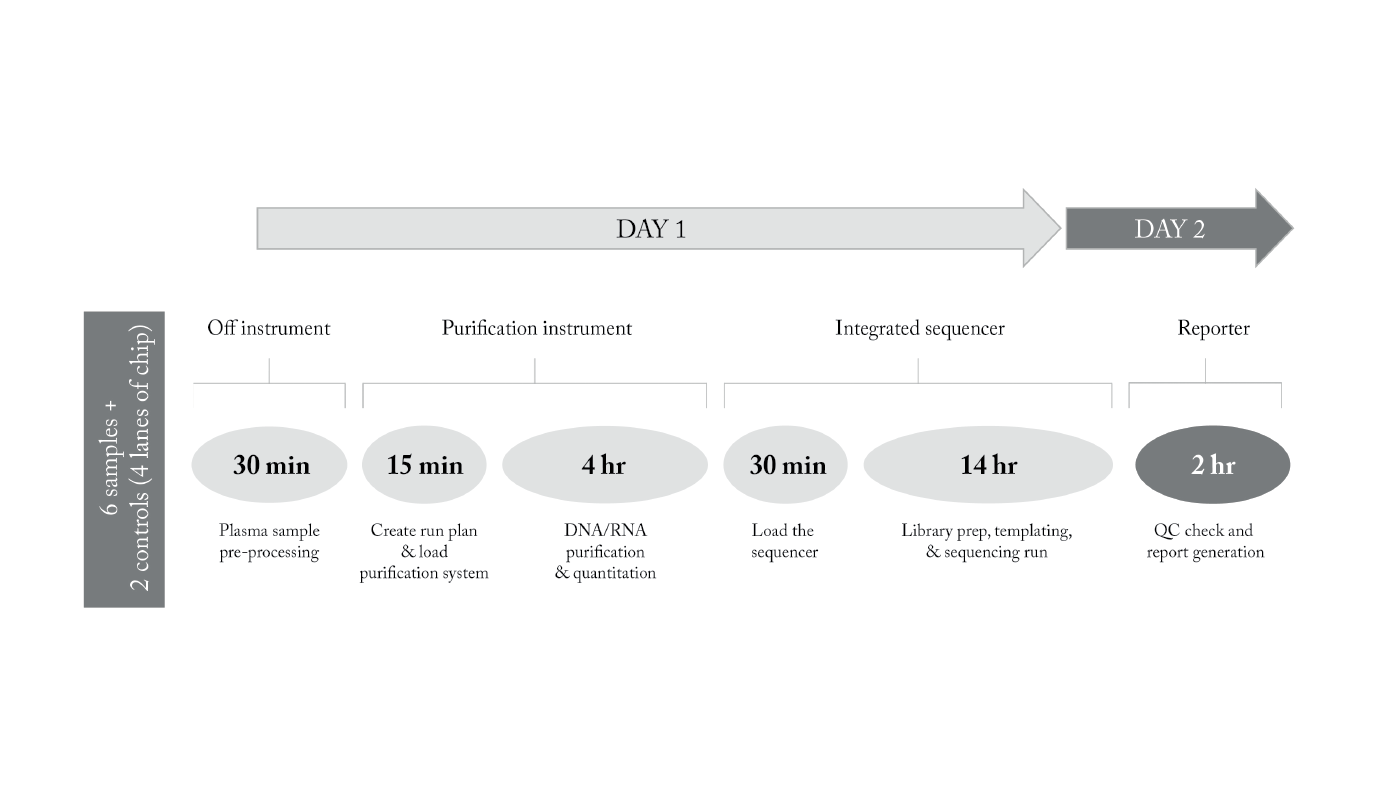
In 2022 we accredited in-house NGS testing of cancer tissue samples to provide rapid and accurate genomic analysis – ultimately enhancing personalized medicine and targeted therapy. And in 2023, we followed this by accrediting NGS testing for cell-free total nucleic acid (cfTNA) analysis from liquid biopsy samples. This minimally invasive technology offers a promising alternative to traditional tissue biopsies, enabling real-time monitoring of cancer mutations and treatment responses.
We achieved accreditation of this service with high sensitivity (>83 percent) and specificity between plasma and tissue and a sequencing LOD of 1.2 percent (at depth of coverage >22 000x). We achieved a 5-day turnaround time for in-house samples, from sample receipt to final report, and developed a service for supplementary testing to tissue samples. This is made possible by our rapid NGS solution, which provides a workflow of just 2 days from plasma sample to report.
Any advice you might have for other laboratories looking to undergo this process?
My advice would be to keep the end goal (ISO 15189 accreditation) in mind as you are implementing and clinically validating the method, and ensure that every step in the development of your service meets the required standards across pre-analytics, analytics, and post-analytics. You need comprehensive understanding of these standards and being prepared to demonstrate compliance in every aspect of your testing will be fundamental to your success.
The journey from NGS naive to a fully accredited NGS facility has been transformative for the service provided by the Department of Pathology at Cork University Hospital. The significant reduction in turnaround times, enhanced sample management, and collaborative opportunities underscore the profound impact of NGS on their diagnostic capabilities. As the field of precision oncology continues to evolve, this hospital stands as a testament to the benefits of embracing advanced technologies, and the importance of continuous learning and adaptation in the pursuit of excellence in patient care.
To find out more about rapid NGS go to https://www.oncomine.com/rapid-lung-ngs-tumor-profiling
Process Maker Review 58487 - 1124





