In the race to digitize pathology workflows, laboratories face a critical decision that will determine their success for years to come: whether to prioritize image quality during the scanning process or settle for post-scan optimization. This choice isn't just about technical specifications – it's about fundamentally reimagining how digital pathology should work.
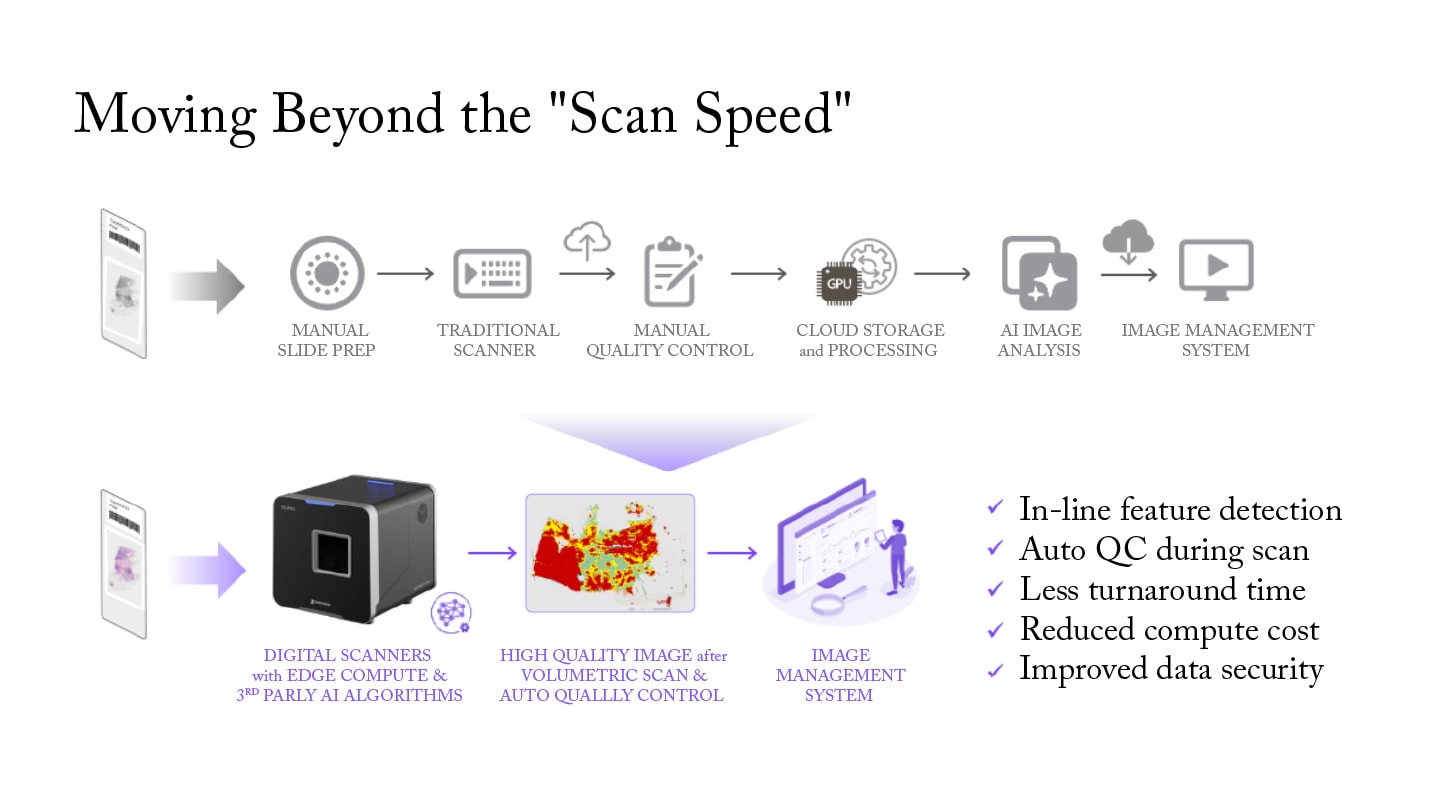
The conventional approach treats image capture and quality assurance as separate processes. Slides are scanned quickly, then reviewed manually for quality issues, with problematic areas flagged for rescanning. Given an industry average of 5% rescan rate, this translates to one in twenty slides being rescanned, significantly increasing turnaround times. This workflow creates bottlenecks, introduces subjectivity, and ultimately compromises diagnostic confidence.
For institutions embarking on their first digital pathology journey, this approach can be detrimental to the advancement of a digital pathology program. Senior pathologists who have spent decades mastering the microscope often approach digital pathology with understandable skepticism. When they encounter suboptimal image quality – blurry areas, poor focus, or missing critical details – it reinforces their belief that digital pathology cannot match the diagnostic precision they've achieved with traditional microscopy. This skepticism can set back programs by months or even years, due to the resulting lack of champions for the adoption of digital pathology.
We think there is a better way; let’s dive in.

The technical imperative: quality at the source
Modern digital pathology systems can now achieve diagnostic-ready image quality during the initial scan through three critical technological innovations working in concert: edge computing, real-time volumetric imaging, and inline scanning algorithms powered by AI.
Edge computing brings sophisticated processing power directly into the scanning system, enabling real-time analysis without the latency and security concerns of cloud-based approaches. This architecture allows the system to make autonomous decisions about focus, exposure, and resolution while the scan is in progress, rather than after the fact. The result is sub-second response times for quality assessment and immediate correction of imaging issues.
Instead of relying on post-scan review, this architecture performs continuous quality evaluation during scanning, analyzing multiple parameters simultaneously to ensure diagnostic-ready images without manual intervention. Advanced AI algorithms powered by edge computing distinguish between actual tissue and artifacts such as pen marks, bubbles, or debris, ensuring complete specimen capture while optimizing scanning parameters for each region.
For particularly challenging use cases, where wet preps, sparse slides, and varying sample thickness create significant digitization hurdles, real-time volumetric imaging can analyze each field of view, mimicking the fine focus of a microscope. This enables the capture of Z-stacks and the fusion of the best pixels from multiple focal planes to produce high-quality images. This Z-stack fusion process combines the sharpest elements from each focal plane, creating a single, fully-focused image that preserves all critical diagnostic details. The system can retain original Z-stacks for further review in areas where AI identifies significant objects, providing pathologists with the depth of information they need for accurate diagnosis.
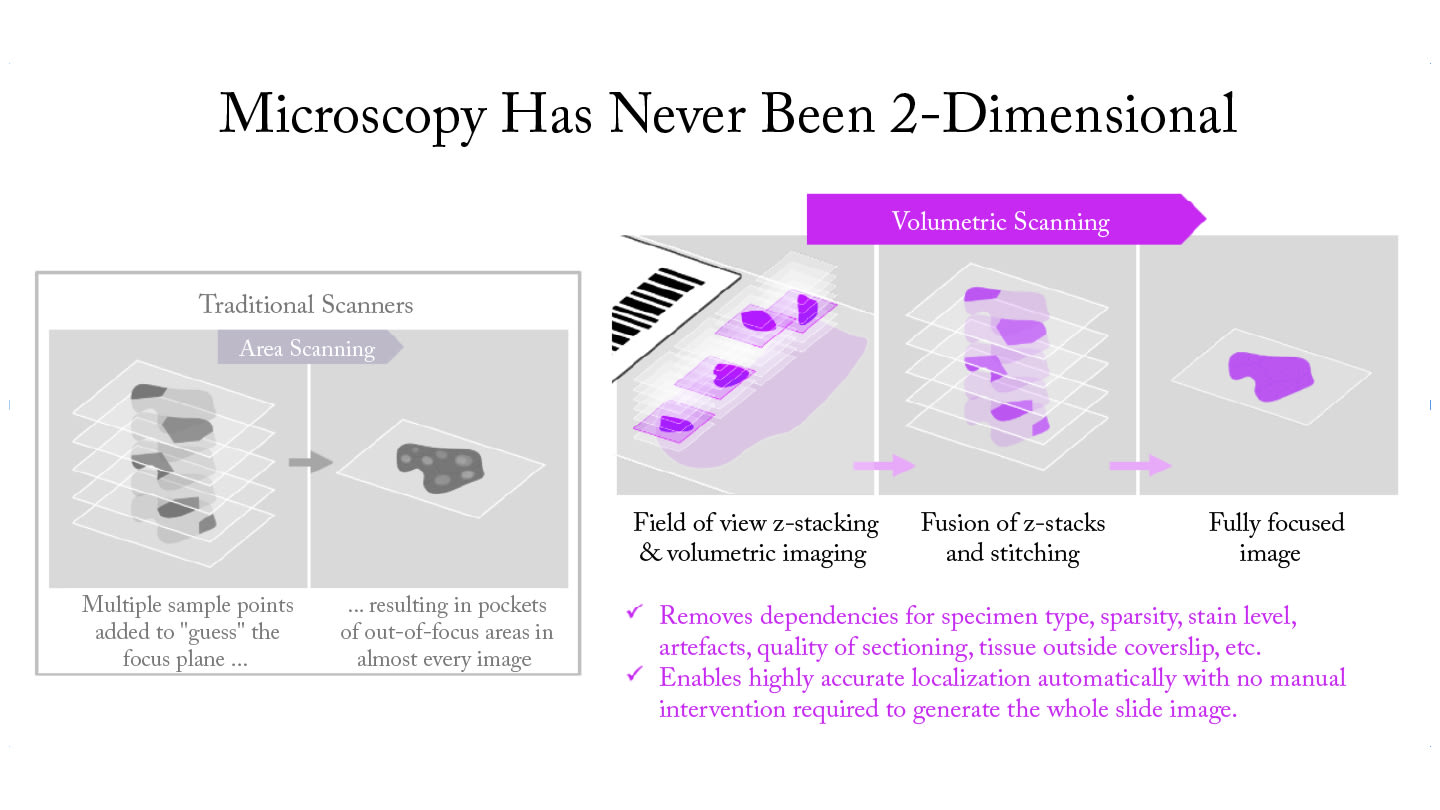
And last but not least, inline scanning algorithms powered by AI adapt to specimen characteristics, automatically adjusting focal planes, resolution, and capture parameters based on tissue type and preparation quality. In sparse Z-stacking, Z-stacks are captured only in areas where inline algorithms detect objects of interest. These algorithms intelligently optimize the number of focal planes captured based on specimen complexity.
This approach eliminates the need for pre-scan preparation while ensuring optimal imaging conditions for each case. The system handles challenging specimens – thick tissue sections, cytology preparations with varying focal planes, and specimens with preparation artifacts – without requiring manual intervention.
The operational reality: why quality changes everything
The impact of achieving quality during scan time extends far beyond technical specifications. It fundamentally transforms laboratory operations in ways that directly affect patient care and organizational efficiency.
Consider two contrasting scenarios that laboratories face when implementing digital pathology:
Scenario 1: The Traditional Approach
A technician loads slides into the scanner and initiates batch processing. After scanning completes, a quality control specialist manually reviews each image, flagging some slides with focus issues, some with missing tissue areas, and some with staining artifacts. Flagged slides must then be retrieved from storage, rescanned, and re-reviewed. The process repeats until all slides meet quality standards. Meanwhile, pathologists wait for cases to be ready for diagnosis, creating a bottleneck that can significantly extend case turnaround times. In some cases, when the quality control staff is overwhelmed, pathologists themselves must perform the final quality assessment – an expensive use of their time that further delays end-to-end processing.
Scenario 2: Quality at the Source
The same set of slides is loaded into an intelligent system that performs real-time quality control during the scan. The system automatically corrects focus issues, identifies missing tissue areas, and adapts to staining variations – all while the scan is in progress. When scanning completes, slides are immediately ready for pathologist review, allowing them to begin diagnosis immediately, reducing case turnaround times from days to hours.
The difference between these approaches is profound. Autonomous quality intelligence embedded directly into the scanning process reduces manual quality control requirements by 90%, allowing staff to focus on higher-value activities. First-pass success rates exceed 99% across all specimen types, with near-zero rejection rates on whole-slide images.
The operational benefits are immediate and measurable. Laboratories implementing this approach report a significant reduction in manual labor, given more than a 20x reduction in slide rescans. As a result, case turnaround times improve dramatically, with consult cases moving from 8-day delays to same-day completion. This results in increases in staff satisfaction as technicians can focus on complex cases and process improvement rather than routine quality control tasks.
With increasing difficulty in recruiting and retaining top talent, labs need solutions that maximize productivity. By automating routine quality control tasks and eliminating the frustration of repetitive rescanning, labs can empower their experienced technicians to dedicate their expertise to higher-value diagnostic work. The dramatic reduction in manual quality control can deliver high throughput, while the improved workflow satisfaction can help attract and retain the skilled professionals who are increasingly difficult to find in today's competitive market.
The technology's ability to handle traditionally difficult specimen types across multiple pathology subspecialties eliminates the operational challenges that have prevented many laboratories from digitizing these critical applications as well. In cytology, where three-dimensional cell clusters and varying focal planes have historically resisted reliable digitization, dynamic Z-stacking technology captures every cell cluster with optimal clarity regardless of its position in the Z-axis. For hematopathology, where nuclear details and chromatin patterns are critical for accurate diagnosis of hematologic malignancies, advanced focus algorithms ensure crisp visualization of cellular details in bone marrow biopsies and peripheral blood smears. In anatomic pathology, the system adapts to specimen variations automatically, handling everything from routine tissues to challenging core biopsies with consistent quality.
Even in microbiology – where slides with varying sample thickness create significant digitization hurdles – the technology can capture Z-stacks and fuse the best pixels to produce high-quality images. By bringing intelligence into the digital scanning process, labs can achieve diagnostic-ready images across all subspecialties, bringing the full benefits of digital pathology to previously underserved areas like parasitology, gram staining, and AFB slides.
The hidden costs of compromising on quality
Organizations that choose to prioritize speed over quality during scan time often underestimate the long-term costs of this decision. The initial savings in scanning time are quickly offset by the operational burden of manual quality control, increased rescan rates, and the diagnostic uncertainty that comes with suboptimal images.
Manual quality control processes introduce subjectivity and variability that can impact diagnostic confidence. This variability creates risk in diagnostic interpretation and can lead to inconsistencies in patient care. Even when dedicated quality control specialists perform this work, the process can remain subjective according to workload, fatigue, or other factors. Pathologists who are forced to perform quality control not only waste their valuable diagnostic time but also introduce additional subjectivity based on their individual diagnostic priorities and experience levels.
The financial impact extends beyond direct operational costs: suboptimal image quality can result in errors, leading to delayed treatment and potential patient harm. The cost of a single diagnostic error far exceeds the investment in quality imaging technology.
Making the right choice
Perhaps most importantly, achieving quality during scan time positions organizations for the future of computational pathology. High-quality images provide optimal training data for AI applications, enabling the development of sophisticated diagnostic algorithms. Real-time analysis capabilities create immediate value while building the foundation for future healthcare applications powered by computational pathology.
This architecture sets the stage for expanded digital adoption in areas like bacteriology, parasitology, and cytology, where object-level resolution in three dimensions can be helpful for diagnostic reads. The ability to run third-party AI algorithms directly on the scanner during the scanning process represents a fundamental shift in how digital pathology systems can be deployed and utilized, making advanced digital pathology accessible to a broader range of laboratories and applications.
For laboratories considering digital pathology implementation or upgrades, the question isn't whether to prioritize quality during scan time – it's how quickly they can make this transition. The organizations that act decisively will gain significant competitive advantages in efficiency, diagnostic confidence, and future readiness.
The choice is clear: invest in quality during scan time now, or pay the price of compromise later. The organizations that choose wisely will find themselves leading the transformation of pathology practice, while those that hesitate will struggle to catch up.
Matthew Wildrick is Senior Director of Solution Architecture, Pramana




