
The prognostic power of spatial proteomics has given rise to a new tool for predicting the recurrence of hepatocellular carcinoma (HCC) after resection surgery. The system, described in a study published in Nature, uses AI to interrogate the distribution of natural killer (NK) cells in the tumor microenvironment and identify high-risk patients.
Here, corresponding author Joe Yeong shares details of the study, the prognostic scoring system that was developed, and its potential impact for patients with liver cancer.
What inspired you and your team to focus on the spatial distribution of immune cells in HCC, and what gaps in current prognostic tools did you aim to address?
The work originally started around early 2021, in response to two emerging trends in HCC management. Firstly, immunotherapy was starting to become the standard of care for late-stage HCC. Secondly, researchers were starting to investigate whether we could identify high-risk patients for adjuvant immunotherapy to prevent or delay relapse.
As an expert in immunotherapy and spatial technology, I wanted to find a way to address that unmet clinical need. I recognized that HCC would be a good candidate for a spatial study, especially for early-stage disease.
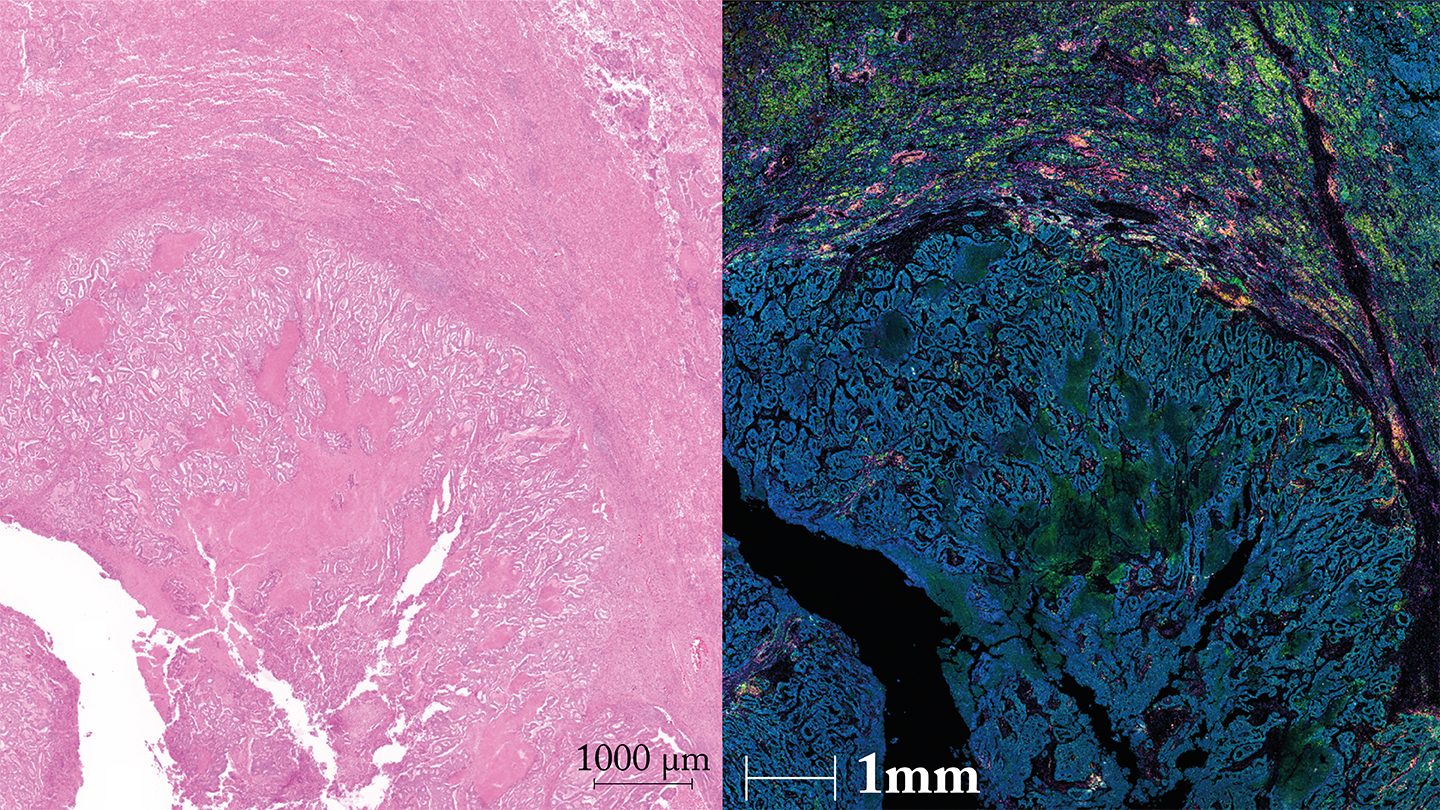
Surgical samples of HCC tumors typically yield sufficient tissue for prognostic testing. Additionally, HCC can be multi-focal, showing well-demarcated regions – stroma (normal), invasive front (border), and tumor center – that allow us to study the dynamic spatial distribution of immune cells.
My team set about using spatial proteomics to answer the question, “Is there a subset of immune cells that infiltrates and penetrates the tumor in a linear fashion toward its center, potentially contributing to better clinical outcomes?”
What were your key findings?
We developed the tumor immune microenvironment spatial (TIMES) score. The AI-powered system leverages spatial proteomics to predict HCC recurrence with 82 percent accuracy.
We also discovered that SPON2+ NK cells at the tumor’s invasive front lower the recurrence risk of HCC by boosting IFN-γ and cytotoxic activity.
How does the TIMES score improve upon existing HCC recurrence risk stratification methods?
TIMES outperformed the tumor node metastasis (TNM) and the Barcelona Clinic live cancer (BCLC) staging systems across early and late HCC stages. Our system also showed stronger disease-free survival association compared with 118 clinical factors – such as vascular invasion – in a 231-patient validation cohort.
I believe the key here is that none of the existing clinical factors include evaluation of the immune status of HCC, when it should be one of the hallmarks of all cancers – including HCC.
What makes NK cells unique as prognostic markers, and how did their spatial distribution influence recurrence outcomes?
NK cell enrichment was significantly higher in the invasive front of non-recurrent HCC compared with recurrent HCC, suggesting a protective role. This was in contrast with other immune cells, which did not show any particular spatial distribution pattern across the adjacent stroma, invasive front, or tumor center.
More importantly, this observation about NK cells can be seen using two different spatial transcriptomics platforms, and two spatial proteomics platforms – multiplex immunofluorescence (mIF) and mass spectrometry.
How does the inclusion of multiple biomarkers, rather than individual expression levels, enhance the predictive power of TIMES?
During the AI training, we found that including both the invasive front and tumor center data was superior to using only the tumor center as an input in terms of prediction accuracy for cancer recurrence.
Similarly we found that including five genetic biomarkers, rather than a single biomarker, resulted in a more accurate prediction model of the TIMES scoring to predict for recurrence.
What are the potential clinical applications of the TIMES scoring system, and how do you envision it being integrated into current HCC management protocols?
Our AI-driven prognostic scoring tool identified high risk patients who might benefit from escalation of treatment. This might be described as the dawn of Spatial Medicine.
There are two possible ways to integrate TIMES scoring into current HCC management. The first involves using a laboratory developed test (LDT) employing mIF to stain the five genes.
However mIF staining is complex and not widely used, so we envision that a better way to clinically implement this is through AI-powered digital pathology. The TIMES score would be predicted based on H&E images of the HCC samples – a concept called H&E 2.0. We are working on the follow-up paper for this – please stay tuned for updates.
What else can we expect from this research?
Immunotherapy is just one of the potential adjuvant regimens for HCC. Further studies are warranted to explore other options to better offer patients personalized management to improve quality of life and survival rates.
We also recognize the potential for different applications of our mass spectrometry-based spatial proteomics technology – which featured in Nature’s Technology of the Year 2024. Our lab is working closely to develop that technology and make it available for a bigger pool of users for more discovery works, including drug discovery for therapeutics leads developments.




