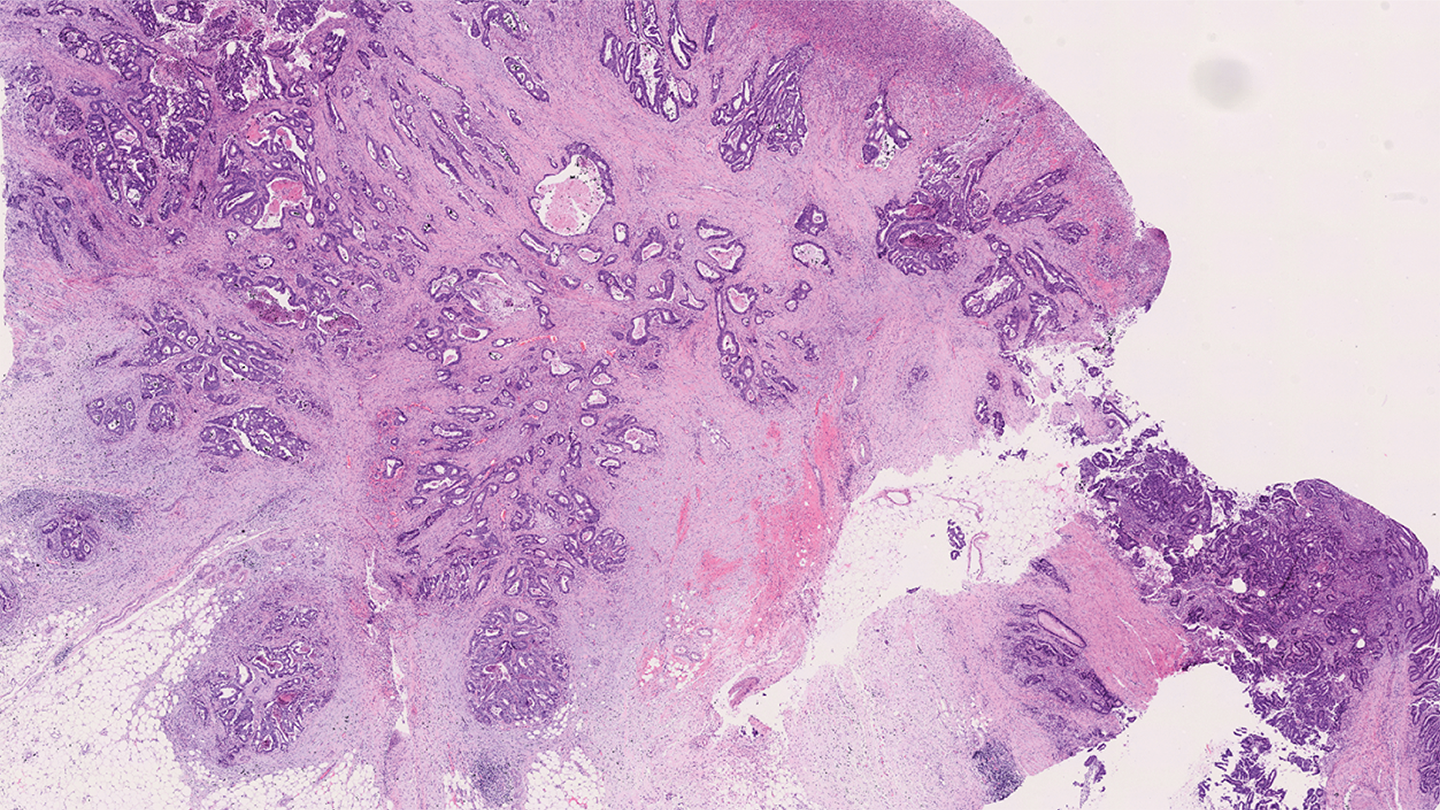
Is clinical decision making due a makeover? In relation to early stage colorectal cancer (CRC), that may be true. The process is complex – around 50 percent of patients are offered chemotherapy after initial surgery when only a small proportion may benefit. So, how can we better identify patients at low and high risk of cancer recurrence and stratify treatment accordingly?
Enter Susan Fotheringham and team, who created OncoProg – an AI-driven clinical decision support tool. We spoke with Fotheringham to learn more about what this initiative could mean for future oncology diagnostics.
What is the technology behind the OncoProg tool?
OncoProg helps patients and oncologists make informed treatment decisions by providing additional clinical information. It is integrated into hospital pathology workflows and clinical treatment pathways.
The system employs digital pathology along with image analysis software to assess two CRC biomarkers – ploidy and stroma:
Ploidy measures DNA content in tumor cells. Normal cells are diploid (two copies of DNA), while abnormal cells are non-diploid (more than two copies). A tumor sample is stained and analyzed to determine DNA content.
Stroma assesses the tumor microenvironment. A low stroma percentage indicates lower recurrence risk, while high stroma (above 50 percent) suggests higher risk. Tumor stroma percentage is determined from an H&E-stained slide.
After surgery, patients are offered the OncoProg test alongside standard tests. The tumor sample is analyzed in a lab, and proprietary software generates a recurrence risk report, categorizing patients into high, intermediate, or low risk. This report is sent to the oncologist and considered alongside other test results to guide treatment decisions. For example, patients at low risk may need surgery alone, while those at high risk may benefit from combination therapies.
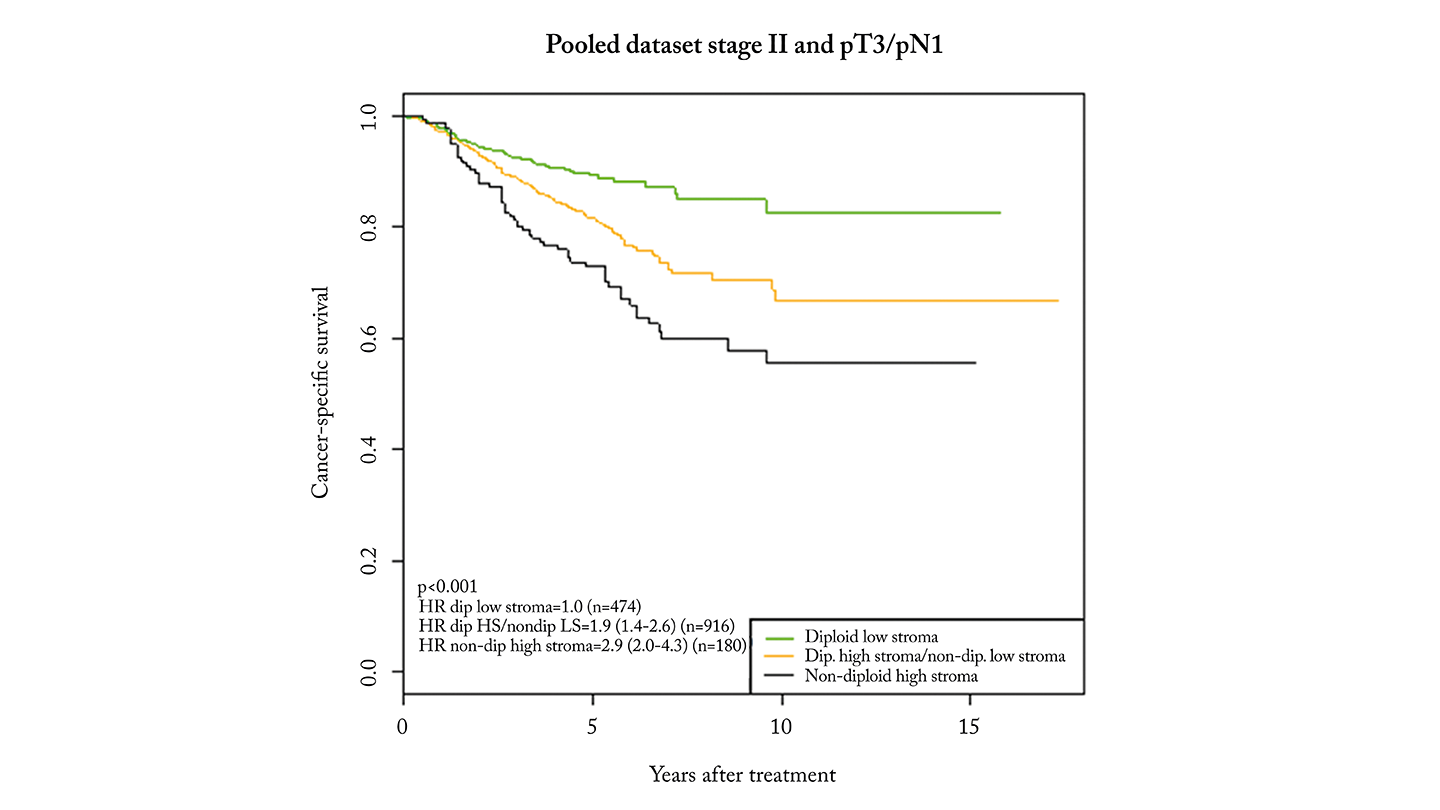
OncoProg was validated in over 1000 stage II patients with CRC in a 2018 study (1). High-risk patients had a threefold higher risk of recurrence compared to low-risk patients. When expanded to include stage II and T3/N1 patients, similar results confirmed its ability to identify those who may benefit from further treatment.

Did you face any challenges during your project, and if so, how did you overcome them?
The first version of the OncoProg test used a specialized microscope to assess ploidy. However, this equipment was not standardized across UK pathology labs, creating a barrier to adoption. To address this, we worked with pathologists and oncologists to create a version that measures ploidy using scanned images, eliminating the need for additional equipment and resources.
A key challenge in developing any digital pathology tool is accessing well-annotated, large-scale clinical trial data for validation. To obtain UKCA/CE certification, OncoProg used a multicenter trial dataset, enabling robust discovery and validation through various data analysis and machine learning techniques.
What is the role of AI in improving diagnostic outcomes with your system?
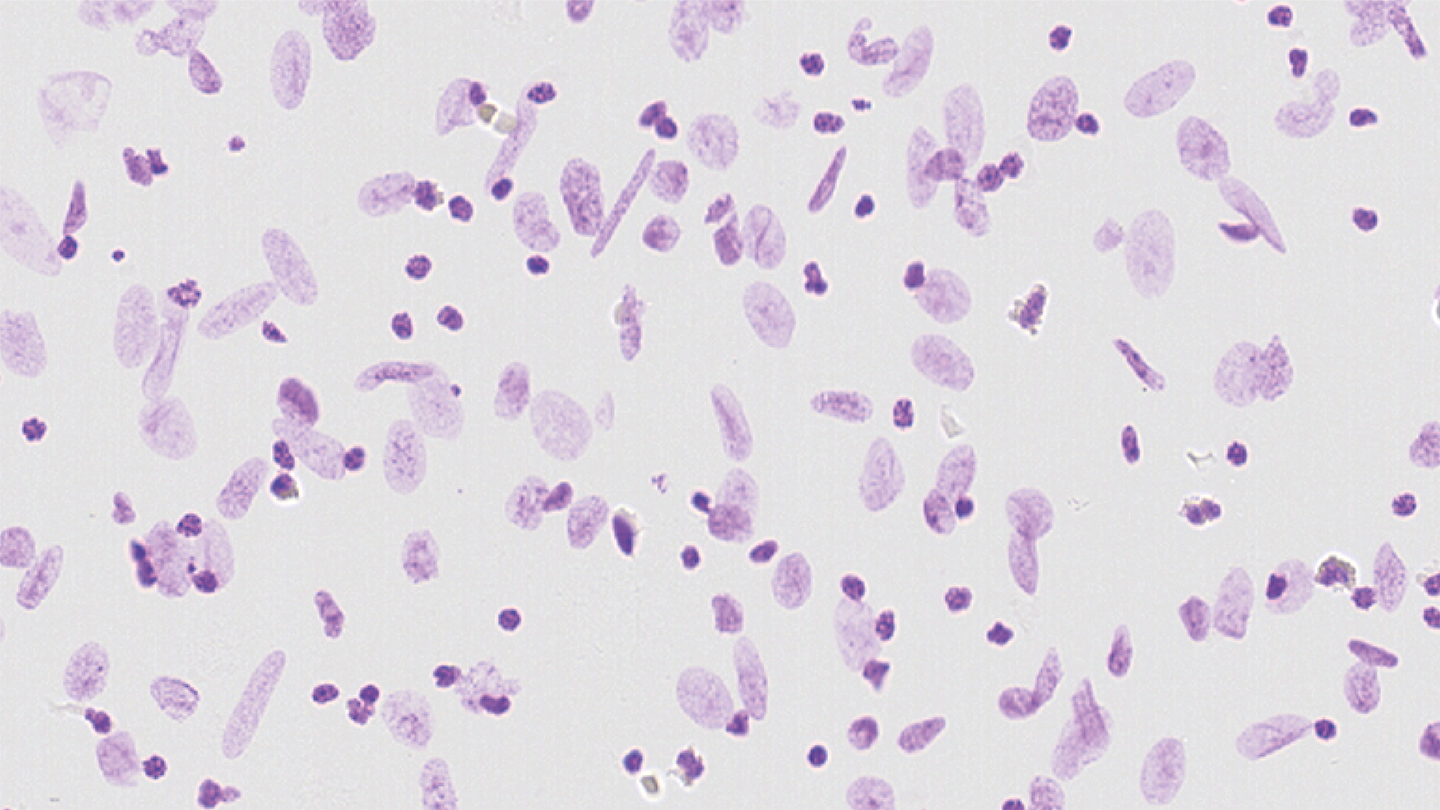
AI is used prominently in two key areas. Firstly, in ploidy analysis, where Ensemble Learning classifies cell nuclei. The image shapes are automatically annotated from the nuclear monolayer slide image using a Random Forest model built on the morphological features.
And secondly, in stroma analysis, where a deep convolutional neural network detects the tumor region in scanned images. This eliminates the need for manual slide annotation by pathologists and fully automates the stroma assessment.
How will you assess the impact of the system on clinical decision making?
We have recently started recruiting for our clinical utility study – prognostic value of ploidy and digital tumor-stromal morphometric analyses for guiding chemotherapy treatment for Stage II / III Colon Cancer Patients (ONCOPROG_AI) – which aims to assess whether the OncoProg test influences decisions about adjuvant chemotherapy for stage II/III colon cancer patients.
The study records treatment decisions before and after providing OncoProg results to see if plans change. For example, deciding against chemotherapy, switching from single-agent to double-agent chemotherapy, or vice versa. It also includes a health economic assessment to evaluate cost-effectiveness and uses questionnaires to measure how the test affects patient and clinician confidence in treatment choices.
OncoProg is already integrated into routine pathology workflows and treatment pathways across several National Health Service (NHS) Trusts in the West Midlands. The study is funded by an NHS AI in Health and Care Award. Recruitment is in the early stages, but initial feedback from oncologists and pathology staff has been positive. We aim to publish the results in early 2026.
How can this technology be effectively implemented into laboratory workflows without disrupting current processes?
The OncoProg software integrates easily into existing NHS pathology lab workflows. It is designed to work within NHS Trusts, with data capture and storage managed through the local network and an OncoProg database.
To ensure seamless adoption, OncoProg is compatible with various histopathology scanners from different manufacturers, eliminating the need for extra hardware – an important consideration in space-limited lab environments.
Are there plans to expand this application to other types of cancer or stages of disease?
OncoProg is a pan-cancer technology with early clinical validation in prostate cancer and assessments in independent international studies. We are collaborating with academic and clinical partners to access well-annotated clinical samples. This will help validate OncoProg for other cancers where early-stage biomarker testing is needed.
Additionally, we’re currently developing a new version of OncoProg, called HistoNav, which eliminates the need for nuclear monolayer preparation in the ploidy workflow. This step is time-consuming, so switching to a method that determines ploidy directly from H&E images will improve efficiency and reduce the workload for pathology labs.
References
- HE Danielsen et al., Ann Oncol, 29, 3, (2018). PMID: 29293881.




