
A new smartphone-enabled, paper-based colorimetric assay solution combines affordability and portability with the precision of modern diagnostics, enabling real-time results in resource-limited settings (1). We connected with Nidhi Menon, head of R&D at HueDx to dig into the details.
Why focus on paper-based assays?
Current methods for clinical chemistry diagnostics are time-consuming, labor-intensive, require exorbitant equipment, and are inaccessible in many regions. Paper-based assays represent an affordable, accessible, and sustainable solution to this problem. These assays are not only cost-effective but also easy to produce and distribute, making them particularly valuable in resource-limited settings.
Additionally, the widespread adoption of smartphones has transformed them into powerful tools for diagnostics. With advanced imaging capabilities, processing power, and connectivity, smartphones offer a versatile and cost-effective alternative to traditional reader systems. This synergy between paper-based assays and smartphones allows for immediate data analysis and results sharing, enhancing the overall efficiency and effectiveness of rapid diagnostic assays. Together, they pave the way for innovative solutions that improve healthcare access and outcomes, especially in underserved communities.

How does your system improve upon other colorimetric point-of-care (POC) methods?
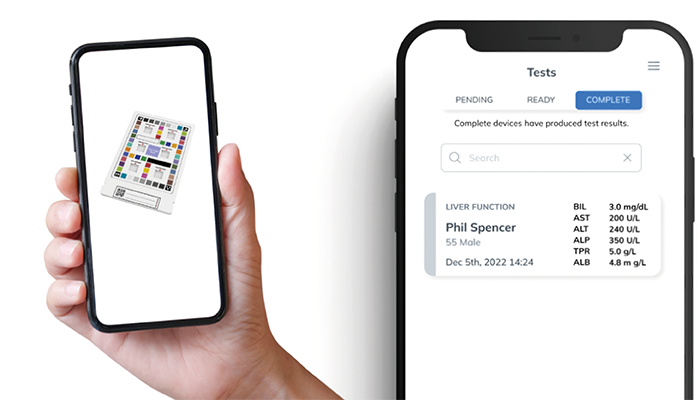
Our system combines colorimetric chemistry with paper-based microfluidic technology and a smartphone app powered by AI and machine learning. At its core is a customizable HueCard, which holds the paper-based assay. The card includes a color sticker to standardize the colorimetric results, ensuring accuracy even with different lighting conditions. This helps improve the precision of analyte concentration measurements, meeting regulatory standards for POC and at-home testing.
Traditional colorimetric POC diagnostics typically provide qualitative or semi-quantitative results, which can limit clinical decisions. Our system advances this by providing truly quantitative results without the need for expensive or complex equipment.
Additionally, the system can be used with existing qualitative tests, allowing them to be adapted for quantitative analysis. This makes diagnostic testing more useful for both patients and healthcare providers, offering clearer and more reliable results in various settings, such as clinics or at home.
Can you share any details behind the development of the assay?
Developing this assay was both exciting and challenging because we were creating a new technology. One of the main challenges was ensuring the color results could be interpreted accurately in any lighting, which can vary significantly. To solve this, we created custom color correction software that adapts to different lighting conditions, making the color changes clear and consistent in any environment.
Another challenge was turning the test into a precise, quantitative tool. This required integrating advanced AI, computer vision, and signal processing to detect subtle color changes and provide accurate measurements. We also needed to make the assay compatible with mobile devices, overcoming limitations in mobile operating systems and hardware. To do this, we developed a custom app that simplifies the image capture process, ensuring reliable results on any device.
Finally, we built a cloud-based system using AWS to securely store images and process results quickly. This infrastructure ensures high-quality data storage and fast analysis, providing users with reliable workflows without compromising result quality.
What are the main barriers to adoption of this technology in low-resource or decentralized environments?
To foster trust, validation and regulatory approval are essential, followed by comprehensive training and education. Additionally, support from governments and non-profit organizations is crucial in low-resource settings. For widespread adoption, raising public awareness and ensuring affordability are paramount.
Smartphone-based assays can be seamlessly integrated with cloud-based services, facilitating a smooth transition into existing health information systems. This integration would enable efficient patient data sharing and management in clinical settings while adhering to safety and privacy regulations.
What are the most significant benefits and challenges of POC diagnostics?
The most significant benefits include rapid test results, enabling healthcare providers to make informed decisions and improve patient outcomes. The low costs, minimally labor-intensive methods, and quick turnaround times would also increase accessibility. Incorporating POC diagnostics in routine laboratory workflows would reduce the burden on laboratory technicians, who can focus on more complex testing.
Some challenges include standardization, quality control, training, implementation, and integration with existing electronic health record (EHR) systems. POC diagnostics must also be thoroughly validated to meet the precision and accuracy required by various regulatory agencies to inform clinical decision making. This will necessitate significant investment prior to integration into routine laboratory workflows.

How do you see POC technologies evolving over the next decade?
I anticipate POC testing will expand to include a broader range of biomarkers and clinical analytes for quantitative testing. Recent advancements in nanotechnology, microfluidics, and the integration of AI and ML have enabled technologies to enhance sensitivity and accuracy of detection and quantitation. Integration with digital health systems will allow for continuous monitoring, enabling patients to advocate for their health and healthcare providers to make faster, informed decisions for improved patient outcomes.
Support in the form of investments in new technologies, public awareness and education, regulatory support, and collaborative networks will be essential for the widespread adoption of POC diagnostics. Financial models and reimbursement policies recognizing the potential of POC diagnostics will be critical in making tests accessible to patients.
Image Credit: HueDx, Inc.
References
- N Menon et al., PLoS One (2024). PMID: 39365798.




