
Spatial biology is offering fresh insights into cancers, neurodegenerative diseases, and autoimmune disorders, and helping researchers identify new biomarkers to improve diagnosis and treatment. But what is spatial image data science – and how does it fit into the picture?
Here, Gourab Chatterjee, Director, Product Strategy and Management, and Lorenz Rognoni, Senior Director, Image Data Science, at Vizgen, guide us through the fundamentals.
What is spatial image data science – and why is it becoming increasingly important in modern pathology?
Spatial image data science uses advanced imaging combined with computational analytics to map and quantify the locations, interactions, and heterogeneity of cells and molecules in tissue.
Its rise in importance is driven by the increasing need to understand complex tissue architecture in diseases. It can provide context that traditional single-marker or bulk measurements can’t offer, ultimately leading to more informed decisions and strategies in therapeutic development.
What unique insights does spatial biology provide over traditional histopathology and genomic approaches in understanding tissue microenvironments?
Spatial biology presents a high-resolution view of tissue architecture and cell-to-cell relationships. Unlike traditional histopathology approaches that predominantly provide morphological details or genomics approaches that mostly examine molecular averages, spatial biology provides quantitative data on cell types, their locations, and interactions.
This spatial image data is particularly valuable in understanding complex diseases like cancer, where the tumor microenvironment plays a critical role in disease progression and treatment response.
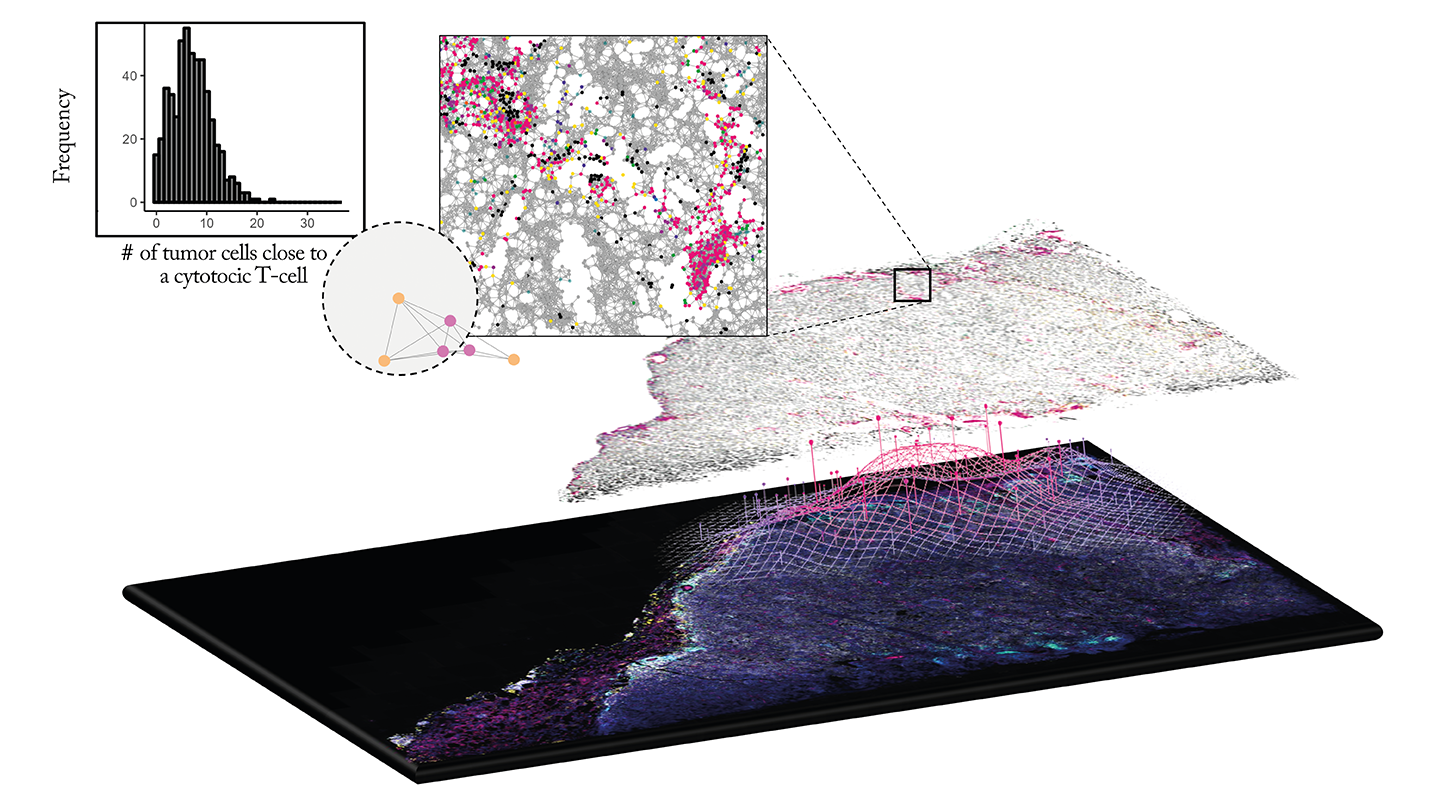
What are the biggest challenges in analyzing spatial image data – and how can pathologists leverage data analytics tools to make the most of this information?
One of the main challenges is managing the volume and complexity of generated data, including storage and processing issues. Additionally, collaborative data access is already difficult. Combining methods to align multiplexed images, standardizing analysis across platforms, and integrating multi-modal datasets are also challenging.
Leveraging advanced data analytics and machine learning tools can help pathologists automate and standardize image processing, quantify features objectively, and integrate spatial insights with molecular and clinical data – ultimately leading to more accurate, reproducible assessments.
How does the integration of multiplexed imaging with spatial analytics improve the accuracy of biomarker discovery and disease characterization?
Multiplexed imaging allows for the simultaneous visualization of numerous biomarkers in a single tissue section. Coupled with spatial analytics, pathologists can identify different cell types and decipher their complex interactions with the microenvironment.
This integrated approach refines biomarker discovery precision and enhances disease characterization. It can uncover subtle shifts in cellular interactions that may indicate early therapeutic resistance or disease progression, such as increased tumor infiltration of cytotoxic T-cells.
What considerations should pathologists keep in mind when interpreting data from spatial imaging platforms?
Pathologists must account for variability in tissue sample preparation, imaging artifacts, and analytics pipelines. The sparse 2D sampling of histology sections should also be acknowledged. It is crucial to validate findings across different modalities and consider each technology’s limitations.
Integrating spatial data with other diagnostic modalities offers a more comprehensive view of the disease, but requires careful correlation and validation to ensure accuracy and relevance.
How is deep learning enhancing spatial image analysis?
Deep learning has greatly improved the analysis of complex spatial patterns in tissue images. It detects subtle cellular differences, recognizes morphological patterns in high-dimensional data, and discovers new features that may be overlooked manually.
In multiplexed immunofluorescence, deep learning-based cell classification allows for threshold-independent positivity classification, which enhances the precision of tissue-based diagnostics by minimizing human error, increasing throughput, improving analysis accuracy, and reducing variability.
Can you provide examples of how AI-powered image analysis has changed the way pathologists assess disease progression or therapeutic response?
AI-powered image analysis has revolutionized pathology by removing rigid thresholds and adapting to tissue and signal variability, allowing for the detection of complex patterns that are challenging to assess manually.
For instance, AI can analyze continuous biomarker data. One model – quantitative continuous scoring (QCS) – was used to assess the normalized membrane ratio for TROP2. This offered nuanced insights into disease progression and therapeutic response in patients with non-small cell lung cancer.
This technology enhances expert analysis by highlighting critical areas in tissue samples and expands access to advanced diagnostics, ultimately improving patient care.
How do you see deep learning shaping the future of digital pathology and personalized medicine?
Deep learning is set to become a cornerstone in the evolution of digital pathology and personalized medicine. As these algorithms advance, they will enhance diagnostic accuracy and integrate multi-modal data – combining imaging, genomic sequences, and clinical information into comprehensive computational models.
In the future, we will see a shift towards fully personalized treatment plans that allow pathologists and clinicians to use data-driven insights to tailor therapies to each patient’s unique disease landscape.




