Speed and Accuracy with Restricted Headcount
Leomar Ballester, Assistant Professor, Molecular Pathology and Neuropathology, and Co-director, Molecular Diagnostics Laboratory, Department of Pathology and Laboratory Medicine, University of Texas Health Science Centre, Houston, Texas, USA.

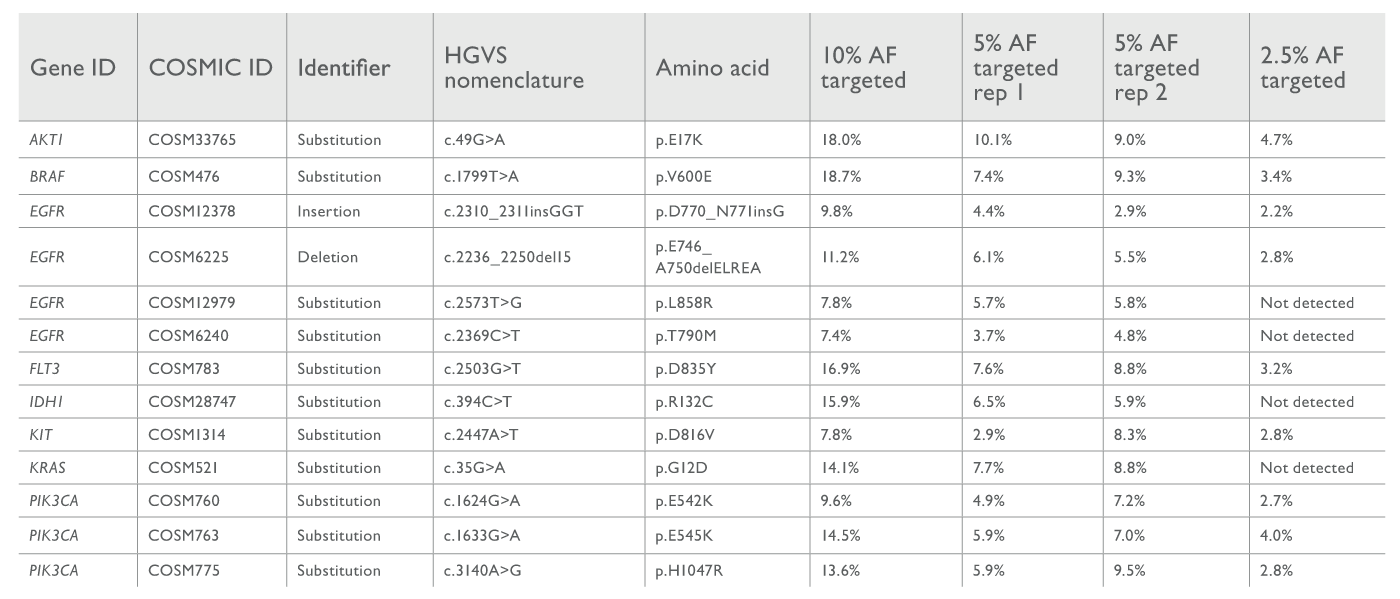
Today’s laboratories must provide accurate results as quickly as possible – often with strictly limited personnel numbers. How robust is the Genexus™ Sequencer in the face of these goals and constraints? To address this question, Leomar Ballester assigned a small team (three individuals) to screen FFPE tissue samples using the Genexus™ Sequencer in combination with the Oncomine Precision Assay. In brief, the team assayed SNVs, CNVs, and fusions with reference to standard commercial controls. The results were excellent; at 5 percent minimal allelic frequency (MAF), the Genexus™-Oncomine system detected 100 percent of variants. Detection sensitivities were particularly encouraging in SNV screens, with 75 percent of SNVs detected at a MAF of 2.5 percent (see Figure 1).
But Genexus™ has other favorable attributes too. Leomar emphasizes the platform’s advantages over competing technologies such as fluorescence in situ hybridization (FISH) or immunohistochemistry (IHC). In particular, he notes the ability of Genexus™ to detect a broad range of genomic variants, including both mutations and amplifications – and, by way of illustration, cites the example of ERBB2 mutations in colon adenocarcinoma. Clinical studies in colorectal carcinoma suggest that over 30 percent of ERBB2 mutations comprise short variant alterations – and these may be detected by Genexus™, but not by IHC or FISH. This is a critical distinction because tumors driven by these short variant alterations may be responsive to targeted therapy; it is therefore vital to patients that we use screening technology that can identify these points of therapeutic intervention. For these reasons, Leomar values the key advantages of Genexus™: speed, accuracy, and a high degree of sophisticated automation. Together, these minimize man-hours while reducing turnaround times to as little as two days.
Streamlined Workflow for Solid Tumor FFPE Analysis
David Seidman, Scientific Director for Molecular Diagnostics and Serology Laboratories, Sentara Healthcare Laboratory Services, Newport News, Virginia, USA.

David Seidman’s laboratory has compared the performance of the Genexus™ technology with that of competing systems and recently presented a head-to-head assessment of the Genexus™ automated workflow technology (Genexus™ Purification System and Genexus™ Integrated Sequencer) and their existing semiautomated workflow system (based on the Ion Chef™ and Ion GeneStudio™ S5 sequencing and semi-automated extraction platforms). Their findings were clear: the Genexus™ technology, by virtue of its unprecedented automation and workflow streamlining, shortens turnaround time from the four days typical of the Ion Chef™/ Ion GeneStudio™ platform, to only two or three days – a time savings of up to 50 percent.
David’s team also undertook a detailed comparison of the Genexus™ Purification System with a competing extraction system, the Promega Maxwell RSC™ 48. Each platform requires some sample pre-processing and has a similar footprint; a key difference, however, is that the Genexus™ platform quantifies both DNA and RNA. Although the Promega technology can process 48 samples in parallel, it can only extract either DNA or RNA. The Genexus™ platform processes 12 samples in parallel, but extracts both DNA and RNA simultaneously, resulting in 24 quantified samples (12 RNA and 12 DNA) per run. Furthermore, the Genexus™ software has built-in flexibility that allows each laboratory to set specific quality control criteria per sample so that successful analysis can be determined with reference to the most appropriate measures for each piece of research. Many laboratories will appreciate this feature. Finally, David notes that the high degree of automation in Genexus™ limits the opportunity for human error; this reduces not only variability in results, but also turnaround time – an important attribute in applications such as cancer diagnosis, where speed is of the essence.
Oncology Biomarker Testing and SARS-CoV-2 Surveillance – a Crisis Combination
Paul Hoffman, Head, Laboratory of Clinical and Experimental Pathology, Louis Pasteur Hospital, Nice, France.
The pandemic has placed unique demands on the laboratory community – primarily with regard to the need for widespread, rapid SARS-CoV-2 testing at high volume. To provide the capacity this unprecedented situation requires, many molecular diagnostics laboratories, including those not traditionally involved in virus surveillance, have diverted resources to either PCR- or sequencingbased SARS-CoV-2 tests. For example, Paul Hoffman’s team – already familiar with the Oncomine Precision Assay for cancer biomarker screening – recently evaluated the Ion AmpliSeqTM SARSCoV-2 Insight Research Panel, used in conjunction with the Genexus™ Integrated Sequencer and Pangolin analysis software. The aim was to determine the platform’s suitability for prospective SARS-CoV-2 surveillance. Can Genexus help us address the pandemic’s unique challenges? Paul’s opinion is unambiguous; he states that the high level of automation in Genexus™ means that fewer personnel are required to maintain workflow, which enables efficient operation without sacrificing social distancing. The automation features also contribute to the ease-of-use of Genexus™ and enable a very rapid turnaround time (~24 hours).
Furthermore, Paul notes that the Genexus Integrated Sequencer allows his lab to process up to 112 samples per week at high quality. The high sensitivity of variant detection makes the Ion AmpliSeq SARS-CoV-2 Research Panel an excellent resource for viral surveillance. In summary, Paul notes the general benefits of NGS advances such as Genexus™ – an approach that permits smaller laboratories to provide genomic profiling services across different fields, thereby enabling them to contribute to the diagnostic challenges raised by the pandemic. In other words, laboratories can divert their expertise to SARS-CoV-2 surveillance without precluding use of the Oncomine Precision Assay for tumor biomarker testing in tissues from patients with a broad range of solid tumors, including lung cancers.
Find out more at www.oncomine.com/ genexus.