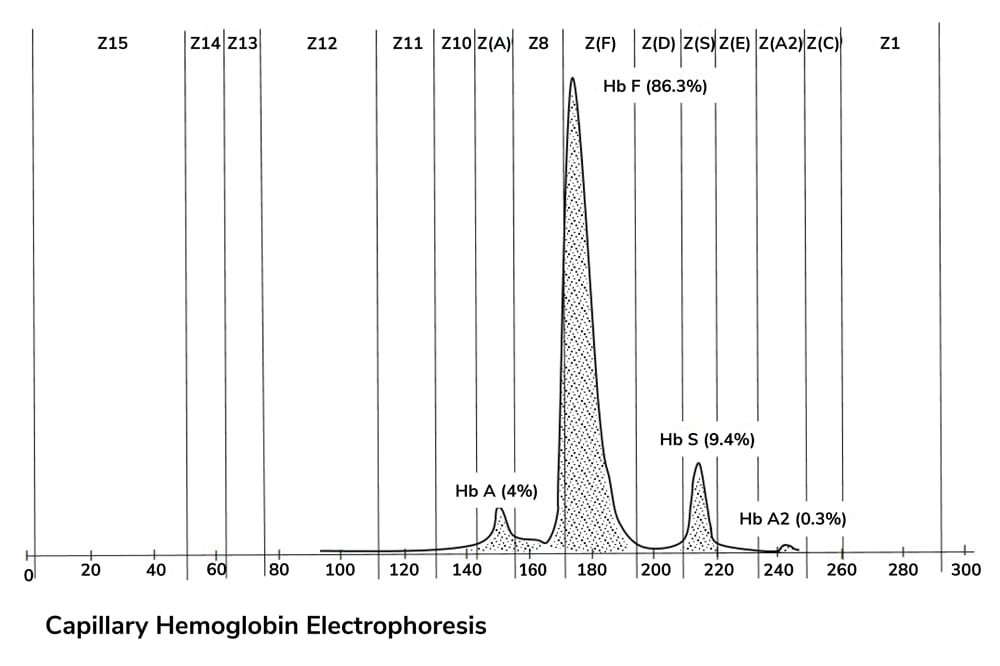
Although the debate over whether or not to screen asymptomatic individuals for prostate cancer rages on, not all testing is equally controversial. In men with advanced prostate cancer, for instance, better testing can improve treatment selection and follow-up care. Scientists from the Institute of Cancer Research (ICR) and the Royal Marsden NHS Foundation Trust have developed a novel, three-in-one blood test that can identify patients for therapy with PARP inhibitor drugs, detect non-responders after treatment initiation, and monitor the cancer itself for signs of evolution and treatment resistance (1).
“By looking at circulating free DNA (cfDNA), we were able to identify mutations linked to responsiveness to olaparib (predictive biomarker), identify within four to eight weeks which patients will benefit substantially (response biomarker), and pick up tumor evolution events leading to drug resistance (resistance biomarker),” explains Joaquin Mateo, Clinical Research Fellow at ICR. The most common changes Mateo and his colleagues see in castration-resistant prostate cancer are in the androgen receptor gene, TP53, and ERG – but he also points out that up to a quarter of patients exhibit defects in homologous recombination genes and may benefit from different therapies. Who is the ideal patient for the new blood test? “We often see patients with bone metastatic disease who have already progressed to two to three lines of standard therapies,” Mateo says. “The tumor may have evolved since the original biopsy, but acquiring tumor tissue repeatedly before a new therapy is challenging. In these patients, cfDNA offers us the possibility for an ‘up-to-date’ genomic screening of the tumor.” Mateo cautions that introducing genomics into patient management is challenging, as it necessitates specialty training – but he thinks it will transform the way prostate cancer is managed. “There are already pathologists, particularly in the United States, who specialize in the use of genomics as a diagnostic or monitoring tool for cancer patients – so why not around the world, and why not in prostate cancer?” The technology still needs to be standardized across laboratories, and the data processing must be simplified – it still requires significant bioinformatics input not normally available outside academic institutions. “This work is already ongoing,” says Mateo. “And certainly, in the next five years, we will see plasma DNA becoming part of the assessment of cancer patients.” He and his colleagues are running a second clinical trial, aiming to recruit up to 90 patients with mutations predisposing them to olaparib sensitivity. By the end of 2017, they hope to have completed the trial and gained a better understanding of how changes in plasma DNA reflect truly functional tumor changes. And, sooner rather than later, they hope to see the assay and analysis methods fully standardized, so that physicians everywhere will be able to use the test to improve and extend their patients’ lives.
References
- J Goodall et al., “Circulating free DNA to guide prostate cancer treatment with PARP inhibition”, Cancer Discov, [Epub ahead of print] (2017). PMID: 28450425.