Improvements in the assessment of acute kidney injury (AKI) risk and associated clinical decision-making is an important goal for healthcare professionals, with recognized room for improvement in the associated diagnostic toolkit. Because we have no specific therapies or cures for AKI, the emphasis is on early diagnosis for earlier intervention. And that begs the question of how we define “early.” The standard biomarker for AKI has long been serum creatinine (SCr), a biomarker for impaired kidney function that only rises once kidney function is more than half compromised; sensitivity is also a recognized issue.
The focus of this article is neutrophil gelatinase-associated lipocalin (NGAL) – a renal biomarker of tubular injury as opposed to loss of kidney function. NGAL levels rise rapidly within a couple of hours of kidney injury (1) thereby offering an opportunity to more effectively detect and exploit the clinical window for intervention. The biomarker is FDA-approved for pediatric use (aged 3 months through 21 years).
AKI - current practice and limitations
The term AKI was first introduced in 2004 to describe acute changes in kidney functionality across a spectrum of severity (2). Higher stages of AKI are synonymous with more traditional diagnoses, such as acute kidney failure, as well as encompassing disorders, such as acute tubular necrosis (ATN); lower stage AKI is indicative of relatively mild dysfunction, but with the potential to escalate. This definition has helped increase awareness of the extent of AKI, highlighting it as a widespread health problem. Often occurring because of – or in combination with – surgical complications and other acute illnesses, such as cirrhosis, sepsis, and acute respiratory disorders, it is estimated to impact 3–20 percent of all hospitalized patients with figures rising to 30–60 percent for those in intensive care (2).
The early stages of AKI are characterized by subtle and predominantly reversible physiological change. Within 72 hours, severe damage sets in, including cell death and intratubular obstruction. The associated health outcomes are correspondingly serious and include increased mortality and increased risk of progression to chronic kidney disease (CKD). The potential for cross-talk between the kidneys and other critical organs exacerbates long-term health risks, particularly for pediatrics. Risk assessment and – where possible – confident detection are essential at an early stage to minimize these more serious outcomes.
In accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) criteria proposed in 2012, AKI is identified and classified on the basis of SCr levels and urine output. However, this approach has significant limitations when it comes to early and specific detection, notably (2,3):
SCr levels are unable to detect AKI at the stage of structural impairment and are not wholly reliable as an indicator of severity.
Elevations in SCr levels lag significantly behind an insult to the kidneys, the concept of renal reserve meaning that around 50 percent of kidney functionality must be lost to trigger the required rise in SCr which as a result typically takes two to three days to occur.
SCr measurements lack specificity – especially with the Jaffe method; but even with enzymatic methods there is a wide range of relevant factors that can impact results from changes in muscle mass, to diet and certain medications.
Evidently, there are inherent flaws associated with the use of SCr with studies suggesting that a SCr-only approach misses at least 25 percent of AKI cases in the patient population as a whole and up to ~65 percent in pediatrics (4, 5, 6).
Understanding NGAL
Renal tubules upregulate and release the NGAL protein in response to stress. Following a kidney injury, NGAL levels rise rapidly, far earlier than SCr and even other AKI biomarkers, such as KIM-1 and IL-18 (see Figure 1). Easy detection in both plasma and urine is a further practical advantage and NGAL has consequently been subject to extensive study. One study in particular analyzed data for more than 16,500 patients across three clinical settings and found that “NGAL significantly improved the prediction of AKI risk over the clinical model alone,” (7).
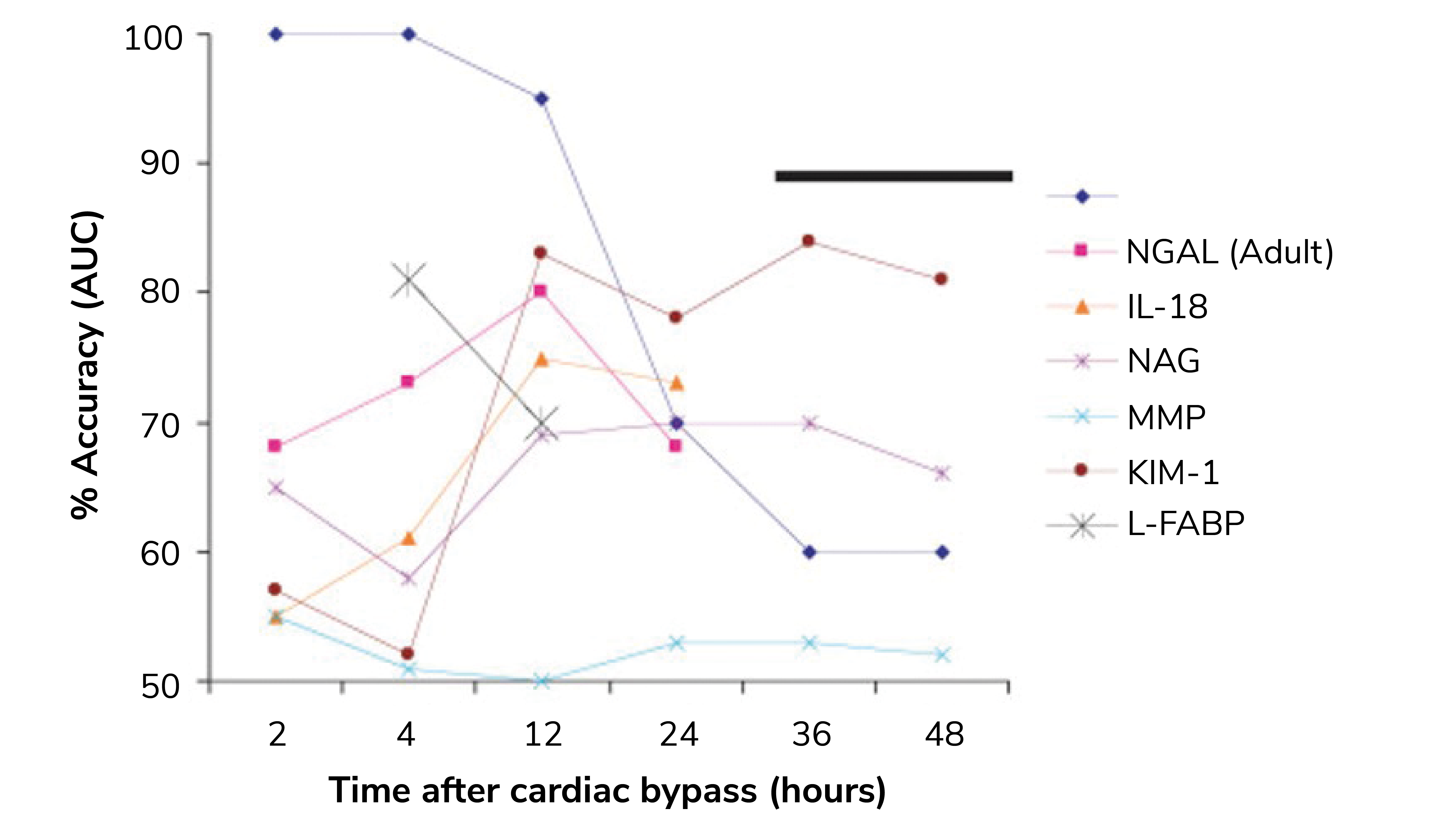
The resulting data provide weighty evidence that NGAL is robustly effective in identifying patients at risk of developing moderate to severe AKI. The use of NGAL to refine clinical decision-making strategies for pediatrics and to differentiate AKI associated with cirrhosis are notable successes that point to broad clinical relevance (9, 10). These studies also indicate that NGAL levels are sufficiently responsive, specific, and proportionate to support effective AKI classification.
The performance of NGAL in pediatric studies is particularly encouraging with a pooled area under the curve (AUC) of 0.90 recorded in studies of post-operative children (11). No biomarker is uniquely specific to any one condition, and we know that age, sex, urinary tract infections, and CKD can all influence NGAL levels, potentially eroding specificity. However, the indications are that NGAL offers significant potential to refine our approach to AKI diagnosis.
The practicalities of implementation
NGAL testing is supported for use in phase 1 clinical trials by both the FDA (12) and EMA (13) and is available “off-the-shelf” thanks to pioneering work by companies.
As lab managers are keenly aware, commercial biomarker tests vary depending on characteristics like analysis time, sample type, and range – all of which influence productivity and uptake. In light of this, the ideal AKI biomarkers:
Are highly reliable assays and easy to use
Deliver analysis in roughly 10 minutes
Offer the ease of a urinary sample as opposed to plasma/serum analysis, even for pediatric and low urine volume patients
Have a wide assay range which is helpful in establishing effective cut-offs for classification and patient stratification in different clinical settings.
The availability of NGAL testing as an open channel assay is also a major plus at a time when fewer vendors are taking this route, because it means that the assay can be easily transferred if the operating platform is changed. The long shelf life of reagents makes it easier to get through periods of low use, typically when the test is first introduced.
This suggests that it’s not the practicalities of testing that present a roadblock to implementation, but rather the practicalities of justifying clinical use. In this regard, one method that is gaining traction with clinical lab leaders is a value-based approach; namely, assessing the potential savings in healthcare costs that NGAL testing might be able to deliver (14).
To see what a value-based approach to NGAL testing might look like, you only have to look at savings estimates at Forest Hills Hospital (14). In a pilot study, AKI was associated with a bump in average length of stay of three to seven days. It was also linked with an increase in per patient costs of between US$4,000 and US$10,000 per day. By implementing an electronic alert based on SCr level measurement, the hospital was able to identify 20 patients a day at risk of AKI and instigate appropriate monitoring to support effective physician intervention. Out of those, 13 percent of identified cases were classified as severe. In the end, the system was estimated to save US$875,000 over a six month period, a level of success that has since been replicated in other hospitals.
These data help to explain the rapid rise of AKI alerting services that are already standard practice in the UK. However, in the trial above, AKI alerts were triggered on average six days after the measurement of baseline SCr levels. It is reasonable to assume that initiating more responsive alerting by adding complementary NGAL testing could further drive down healthcare costs.
Integrating NGAL into clinical decision-making algorithms
In a year-long study at Cincinnati Children’s Hospital Medical Center, an NGAL assay was used to improve the prediction of AKI in a pediatric ICU with a patient population ranging from child to young adult (9). The results show that using the clinical decision support algorithm shown in Figure 2 substantially improves AKI risk assessment relative to the use of a renal angina index (RAI) based on demographic risk and physiological change (SCr and positive fluid accumulation) that Goldstein et al have been validating for the past ten years.
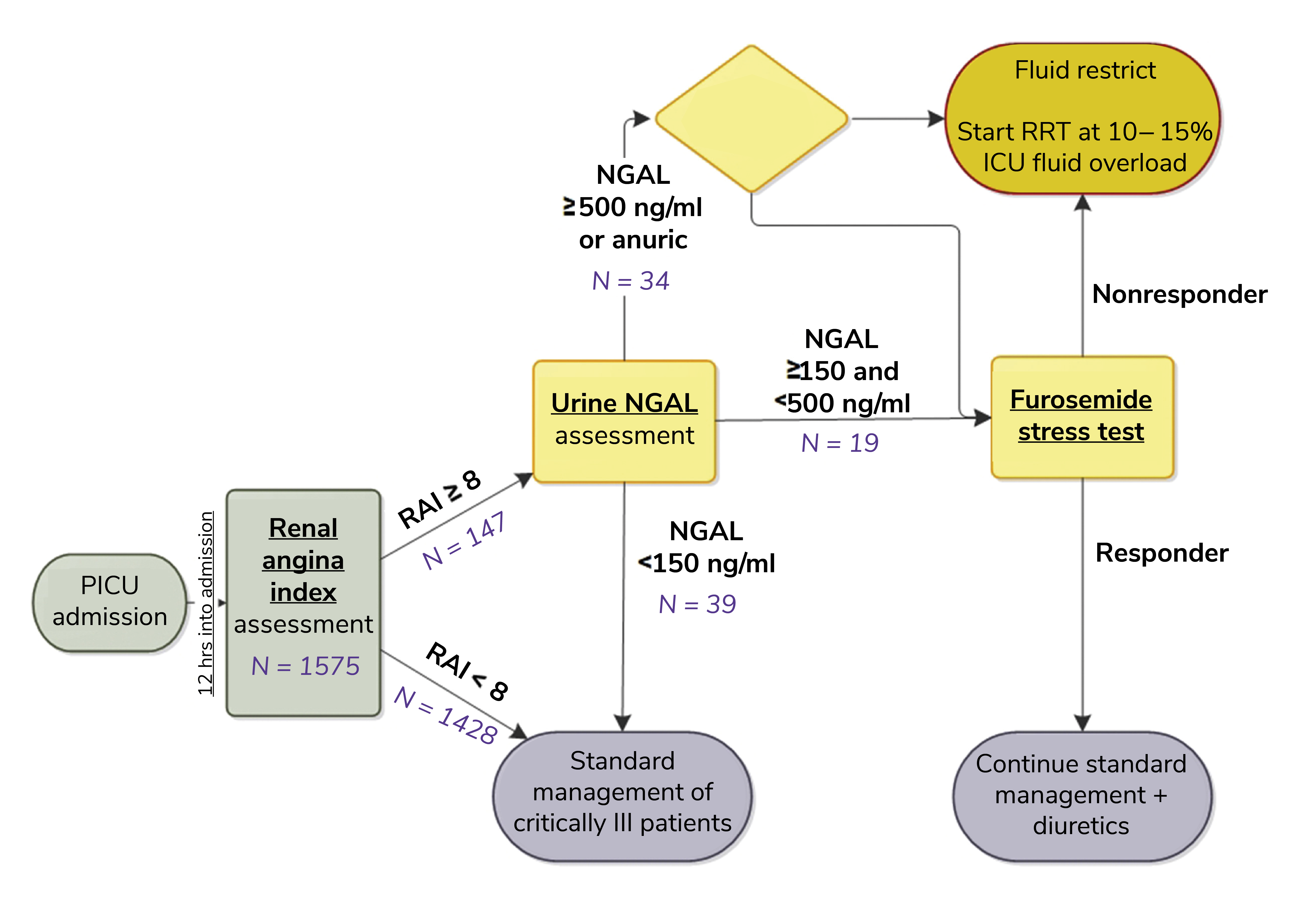
The RAI already performs well with respect to the prediction of AKI at 72 hours, with an AUC-ROC of 0.88 observed in a multiple study review. However, this response was improved to 0.97 by the inclusion of urinary NGAL assessment for patients registering an RAI ≥ 8. Furthermore, the positive predictive value (PPV) of 0.37 for the RAI alone was improved to 0.64 via the inclusion of NGAL, with no deterioration in negative predictive value (0.98 in both cases) (9).
An important lesson to draw from this work is that NGAL is a complementary test rather than a replacement one. In the assessed workflow, the RAI is used to determine which patients admitted to the ICU are at highest risk of AKI and would therefore benefit from an NGAL test, focusing on the use of the biomarker. This strategy is effective for reducing costs, improving performance, and maximizing confidence – all critical when promoting modified clinical practice.
Further compelling evidence of the value of NGAL testing comes from a study of a very different patient population: adults presenting with decompensated cirrhosis (10). AKI manifests routinely in cirrhosis patients in the form of either prerenal AKI, hepatorenal syndrome (HRS), or acute tubular necrosis (ATN). Differentiating AKI type is challenging, notably for HRS and ATN, but important for optimal care. Here, SCr levels provide minimal insight but NGAL has shown considerable promise. This study was designed to explore that potential, focusing on the ability of NGAL to differentiate ATN from non-ATN AKI and to support the prediction of 90-day mortality.
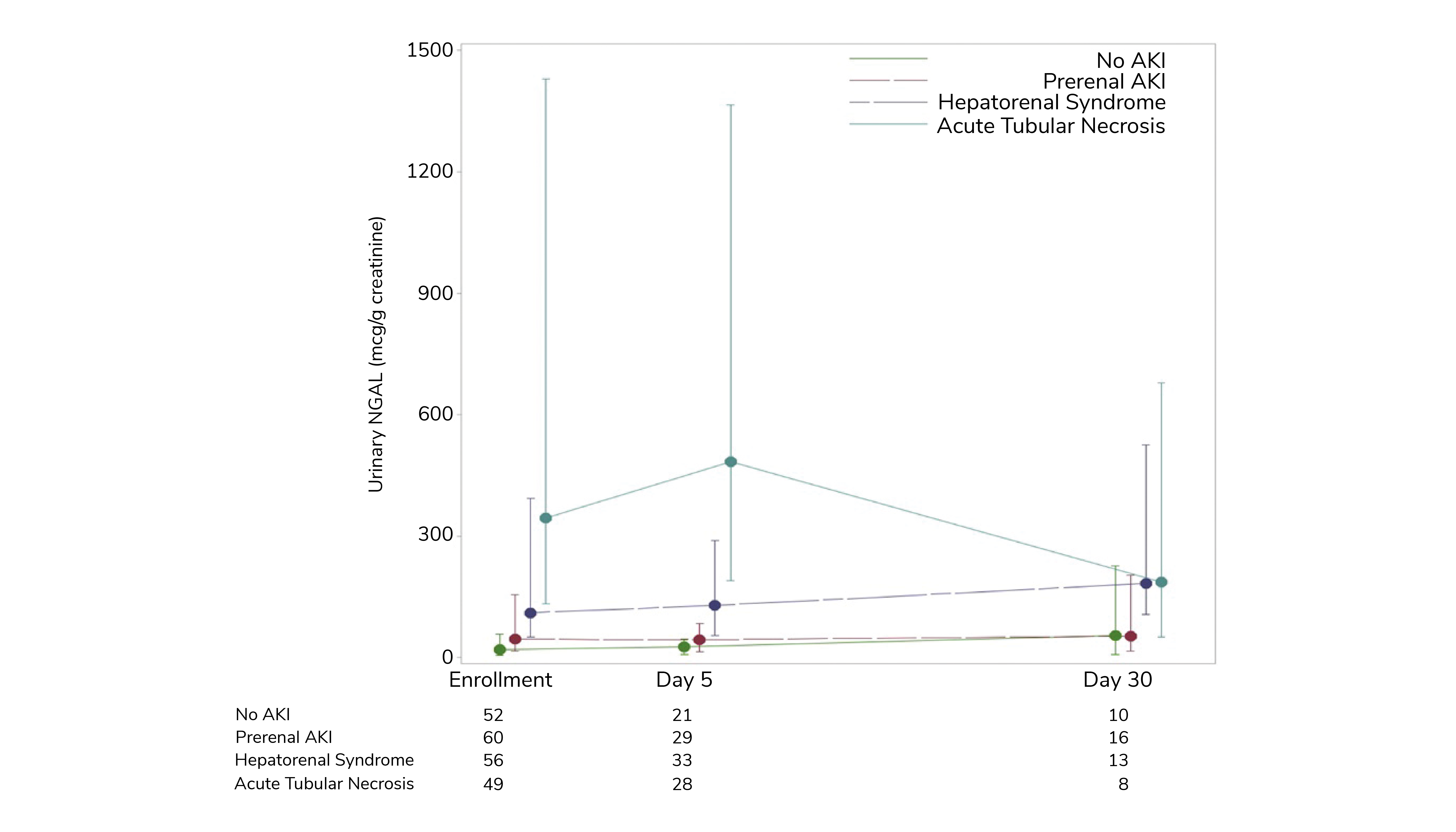
Figure 3 shows urinary NGAL data for a total cohort of 213 hospitalized patients at enrollment and for a subsection of the population at two points during the course of a month. These results clearly differentiate between the types of AKI. The absence of AKI and prerenal AKI are distinguished by consistently low NGAL values; the highest values are observed in patients with ATN. This study is the largest yet carried out in the US using NGAL in this clinical environment, but the results align with those from a similar study in Barcelona (11). They provide evidence of the clinical potential of NGAL to augment the current diagnostic toolkit for identifying AKI in cirrhosis patients and for implementing an appropriate therapeutic response.
Challenges and future directions
The speed with which NGAL levels change in response to both deterioration or improvement in the kidney, coupled with our ability to detect such changes rapidly and reliably, provides a platform for substantial improvement in the treatment of AKI. NGAL testing introduces opportunities for enhanced intervention on the basis of close to real-time impact monitoring and to explore NGAL-directed therapeutics to improve AKI prognoses. It is exciting to think that it could help us maximize treatment efficacy, while reducing cost and minimizing adverse outcomes.
Allegretti and colleagues (10) noted that, in addition to differentiating ATN and HRS, low NGAL levels were potentially valuable in identifying HRS patients with “relatively preserved renal parenchyma,” who, by extension, would have a greater likelihood of responding well to vasoconstrictor therapy. Conversely, higher median NGAL values were associated with death at 90 days in this study, with the addition of NGAL testing enhancing the performance of established prognostic models for predicting 90-day transplant-free survival and 90-day survival improved performance in all cases (model for end-stage liver disease [MELD], MELD-Na, and chronic liver failure consortium acute-on-chronic liver failure [CLIF-C ACLF]). For the Goldstein team (10), a signposted next step is to focus clinical intervention on patients that register both a high NGAL and RAI result to determine the extent to which outcomes can be improved for this most vulnerable cohort.
Realizing this potential will require greater clinical familiarity with the NGAL assays and the establishment of effective cut-off values to support clinical decision-making in different settings. The diversity of clinical settings in which AKI presents calls for dedicated work across multiple patient populations, but compelling progress is already being made in many areas. Evidence gathered to date indicates that wherever AKI presents, there is significant opportunity for NGAL testing to improve our response.
What lies ahead?
Though SCr and urinary output remain vital criteria for the detection of AKI, few would argue with the need for better diagnostic tools and practice. The speed, sensitivity, and specificity of NGAL marks it as a promising catalyst for change. Using NGAL testing, we could help clinicians redefine “early intervention” from days to hours – and reap the associated rewards. Collectively, we could develop a robust biochemical AKI definition to stimulate targeted drug development and standardize best-practice decision-making. By using NGAL test data through automated, electronic alerting and advice, we can envision almost real-time detection, monitoring, and support before AKI progresses.
The widespread nature of AKI, the associated healthcare costs, and the long-term sequelae suggest ample rewards that could easily justify the costs associated with the implementation of NGAL testing. As reliable, easy-to-use tests become available, we should encourage their clinical adoption.
References
- CD Krawczeski et al., “Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass,” J Am Coll Cardiol, 58, 2301 (2011). PMID: 22093507.
- K Makris, “The role of the clinical laboratory in the detection and monitoring of acute kidney injury,” J Lab Precis Med, 3 (2018).
- JP Roy et al., “Under-recognition of Neonatal Acute Kidney Injury and Lack of Follow-up,” Am J Perinatol, 39, 526 (2022). PMID: 32971562.
- A Kaddourah et al., “AWARE Investigators. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults,” N Engl J Med, 376, 11 (2017). PMID: 27959707.
- N Stanski et al., “Integration of urinary neutrophil gelatinase-associated lipocalin with serum creatinine delineates acute kidney injury phenotypes in critically ill children,” J Crit Care, 53, 1 (2019). PMID: 31174170.
- Z Ricci et al., “Acute Kidney Injury in Pediatric Cardiac Intensive Care Children: Not All Admissions Are Equal: A Retrospective Study,” J Cardiothorac Vasc Anesth, 36, 699 (2022). PMID: 33994318.
- A Haase-Fielitz et al., “Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status,” Ann Clin Biochem, 51, 335 (2014). PMID: 24518531.
- J M Thurman and C R Parikh, “Peeking into the black box: New biomarkers for acute kidney injury,” Kidney Int, 73, 4 (2008). PMID: 18235520.
- SL Goldstein et al., “Integration of the Renal Angina Index and Urine Neutrophil Gelatinase-Associated Lipocalin Improves Severe Acute Kidney Injury Prediction in Critically Ill Children and Young Adults,” "Kidney Int Rep, 7, 1842 (2022). PMID: 35967111.
- AS Allegretti et al., “Urinary NGAL as a Diagnostic and Prognostic Marker for Acute Kidney Injury in Cirrhosis: A Prospective Study,” Clin Transl Gastroenterol, 12, 5 (2021) PMID: 33979307.
- I Sandokji, JH Greenberg, “Novel biomarkers of acute kidney injury in children: an update on recent findings,” Curr Opin Pediatr, 32. 354 (2020). PMID: 32332324.
- FDA (2016). Available at: https://bit.ly/3HmbOHY.
- EMA (2016). Available at: https://bit.ly/425Yb9h.
- P Huelin et al., “Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis: A prospective study,” Hepatol, 70, 319 (2019). PMID: 30810244.




