EVs for Ovarian Cancer Diagnosis
Three proteins identified as biomarkers for high-grade serous ovarian carcinoma
Georgia Hulme | | News
Ovarian cancer is the fifth most common cancer in US women, yet early diagnosis remains a global health care challenge. Often dubbed “the silent killer,” its largely asymptomatic nature means that only 20 percent of ovarian cancers are found early, and there are no national screening programs available because of the unreliability and ineffectiveness of current tests (1). With that in mind, researchers from Nagoya University, Japan, have turned to ovarian cancer extracellular vesicles (EVs) – including exosomes – as promising biomarkers (2).
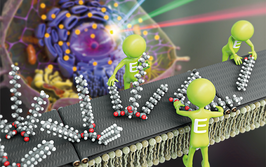
“We revealed the detailed protein information on ovarian cancer EVs and their diversity,” says Akira Yokoi, Assistant Professor of Obstetrics and Gynecology at Nagoya University and lead author of the study. The research, which focused on identifying specific membrane proteins for high-grade serous ovarian carcinoma (HGSOC), used liquid chromatography-tandem mass spectrometry to analyze the proteins contained in small, medium, and large EVs. “Originally, the challenge was to find ovarian cancer-specific proteins in ovarian cancer EVs. We tested multiple methods, including unique ELISA for EV detection, western blotting or referring database for discovering targets and validating them,” says Yokoi. They found that small EVs were more effective biomarkers than medium and large ones, and identified FR-alpha, Claudin-3, and TACSTD2 as proteins closely associated with HGSOC.
The team also developed a novel method of simple EV isolation using polyketone-coated nanowires (pNW). Indeed, the platform successfully purifies EVs from biofluids, making it well-suited for clinical application. The paper suggests “broad potential for pNW-based applications for isolating further specific EVs in circulating body fluids.”
The one thing Yokoi wants laboratory medicine professionals to take away from these findings? “Deep understanding of EV biology can lead us to new applications in the clinic.” The team aims to validate the performance of the identified biomarkers in clinical trials, and, in the future, they hope to apply this system to ovarian cancer screenings.
- American Cancer Society (2023). Available at: http://bit.ly/3OnXaEx
- Akira Yokoi et al., Sci Adv, 9 (2023). PMID: 37418532.
Associate Editor for the Pathologist