Science That Lasts a Lifetime
Yassmine Akkari discusses the evolution of cytogenetics – and its timeless importance
Yassmine Akkari | | 4 min read | Opinion

For decades, cytogenetics has been declared dead. But for as long as I can remember, I have never understood this statement. In my mind, cytogenetics is the science of chromosomes – and how can a science die?
A quick google search brings up many different definitions of the word “cytogenetics.” These include “the study of inheritance in relation to the structure and function of chromosomes,” “a branch of genetics concerned with how the chromosomes relate to cell behavior, particularly during mitosis and meiosis,” and “a branch of biology focused on the study of chromosomes and their inheritance.” Nowhere in these definitions does it imply that this area of genetics is linked to a particular technology or that it has a limited lifetime.
While discussing this matter with colleagues, it became apparent that the concern over the “longevity” of cytogenetics lies in the fact that genetics has progressed into genomics, and by focusing on sequence variation and advanced sequencing technologies, we have neglected the principle of chromosome science and the importance of understanding chromosome behavior.
The technologies that we use to interrogate chromosome structure have evolved throughout history and the testing landscape of cytogenetics has changed dramatically. Cytogenetics has taught us that there are 46 chromosomes in normal human cells and has allowed us to distinguish chromosomes based on their banding patterns. These advances legitimized the birth of clinical cytogenetics and acknowledged the burden of chromosome aberrations in human disease. Just as the detection of single nucleotide variants has evolved from Sanger sequencing to next-generation sequencing, the detection of chromosome alterations has evolved from G-banding to FISH to chromosomal microarrays to – perhaps in the very near future – optical genome mapping (OGM) and whole genome sequencing (WGS) (see Figure 1).
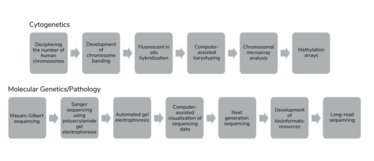
Figure 1
Our genomic community now faces a lack of understanding on the behavior of DNA in health and disease at the chromosome level – the view at 30,000 feet. We are experiencing a decline in interest from future geneticists on the importance of understanding this science.
Why? Because it appears that looking at a G-banded karyogram is an archaic practice and doesn’t allow for the single-nucleotide resolution that is afforded by advanced molecular methods. The real question is: Are all human diseases driven by single nucleotide variation? The answer is no. Is G-banding a technology that allows for a well-established view of the genome at a single-cell level? The answer is yes. So, what’s the problem?
The problem is that we didn’t fight to continue education on the science of cytogenetics. In 1993, the human genome project promised the field an unprecedented understanding of human health and an explosion of interest occurred in genomic sequencing. Though this project advanced the discovery of the genes involved in mendelian disorders, there was also a parallel line of discoveries in the world of oncology: targeted therapy. These two waves of genomic advances allowed for interventional clinical management and therapies for both constitutional and somatic disease, and enriched the medical community with molecular technology-focused teaching curricula. While this legitimized genomics as a bona fide medical specialty, cytogenetics was overlooked. We started experiencing a shortage in professionals who had expertise in cytogenetics and witnessed the decrease in national programs dedicated to cytogenetics training. This, along with the rumor that cytogenetics was dead, discouraged younger generations from receiving training in the field, further accentuating the lack of innovation and appreciation (see Figure 2).
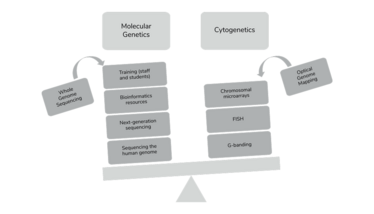
Figure 2
Ironically, despite a decrease in the number of cytogenetic trainees (both at the director and technologist level), the workload in cytogenetic laboratories never wavered. Many had predicted a decrease in prenatal cytogenetic workload because of the wide adoption of non-invasive prenatal screening or a decrease in cytogenetic analysis of a child in the NICU. These changes did not materialize. Further, in this era of limited healthcare resources, do we really need to perform WGS to confirm the diagnosis of Down syndrome?
Slowly but surely, we have started to realize that a good molecular geneticist/pathologist needs to fully understand chromosome structure and function. As we start to derive information about chromosome copy number and structural rearrangements from sequencing technologies, we are becoming more cognizant of the need to learn cytogenetics. For example, with NGS sequencing, it is important to appreciate that, if one sees a deletion on one end of a chromosome and a duplication at the end of another chromosome in a child with congenital anomalies, it may be an unbalanced recombinant from a balanced translocation. It is crucial to understand and schematically visualize the preceding meiotic event. Why? Because it will have a profound impact on our ability to provide accurate determination of recurrence risk.
Certainly, we must pause and appreciate the immense advancement in genomics. This progress touches on sequence variation, chromosomal structural rearrangements, epigenetic processes, and the effect of clonal heterogeneity. But it is equally important to understand that cytogenetic technologies are also evolving, and that the implementation of the new and upcoming OGM and WGS will benefit from cytogenetic interpretation.
In conclusion, cytogenetics remains extremely important, and reciprocal training and education on both ends of the DNA technology spectrum (whole chromosomes to methylation and single nucleotide aberrations) will allow true breakthroughs in genomic science.
Senior Director, Clinical Laboratory, The Steve and Cindy Rasmussen Institute for Genomic Medicine, Nationwide Children’s Hospital and Professor, Department of Pathology, The Ohio State University College of Medicine, USA