Avoiding Titanic Errors
The preanalytical phase is subject to more error than any other part of the testing cycle – what can we do to improve it?
Preventable medical errors are not something that people working in healthcare really want to think about, but they are a reality and the numbers aren’t small. A recent report estimates mortality resulting from medical error in the US alone to be more than 400,000 deaths per year (1). According to WHO, one in 10 patients suffer from some kind of error during hospitalization in developed countries (2) and 8–12 percent of patients in EU countries are thought to experience preventable adverse events (3); unsurprisingly a huge spotlight has been cast over patient safety, placing it as an issue of concern by the European Commission.
Given the importance of laboratory tests on the overall medical decision-making process, laboratory errors make a key contribution to the overall risk of error in healthcare. In a separate study that looked at the frequency of diagnostic errors, the testing phase (failure to order, report, and follow-up laboratory results) were found to contribute to the majority of diagnostic errors (44 percent) (4). This number is unacceptably high. Like any clinical or diagnostic field, laboratory medicine is subject to error. While this may not come as a surprise to anyone, it’s less well known that most of this error occurs during the preanalytical phase (5), which includes the collection and handling of diagnostic specimens. Because most of these problems are preventable through effective quality control, the European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) has set up a working group to focus exclusively on preanalytical phase quality (WG-PA) (see “For the Greater Good”).
For the Greater Good
Who? Working Group for Preanalytical Phase (WG-PA) created by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM)
When? Formed in 2012
Who? WG-PA has three full members, one young member, several corresponding members from different European countries, and two expert consultants from industry
Why? All members share the goal of harmonizing and improving quality standards for the preanalytical phase. It’s a goal that breaks down into several parts:
- to promote the importance of preanalytical phase quality across Europe
- to assess current preanalytical phase practices and policies in European laboratories, identifying the most critical problems and issues for improvement
- to produce recommendations and provide guidance for implementation across Europe
- to educate laboratory professionals by providing educational materials, organizing conferences, courses, webinars, and more
- to encourage harmonization of preanalytical practices in all European countries
All errors are not equal
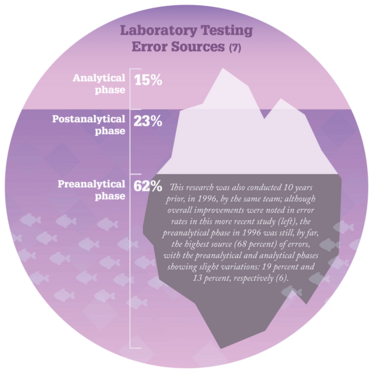
Why is it so important to ensure quality and harmony during sample collection and handling? Numerous studies (6,7) suggest that nearly two-thirds of all laboratory error occurs in the preanalytical phase (see Figure “Laboratory Testing Error Sources”). Why? First of all, there are not many standards for safe, patient-centered and evidence-based preanalytical practices – and those that do exist are often not evidence-based. Second, many steps in that phase (for example, test selection, patient preparation, patient identification, sample collection and delivery to the lab) are performed manually by non-laboratory staff, outside the direct supervision of laboratory professionals. And finally, stakeholders involved in this part of the testing cycle often lack not only the necessary training to conduct the tests, but also an understanding of the procedures they are performing and how their actions affect sample quality and patient results. In one recent study (8), we showed that blood sampling across Europe is done by members of different professions with different education, background, competence, and skill levels. In some countries, even administrative staff are involved in venous blood sampling! All of these factors contribute to the high potential for error in the preanalytical phase.
Not all errors are created equal, though. Some are made more frequently than others, and some carry a greater risk of harm to the patient. When thinking about ways to reduce errors, your first step should be a careful risk assessment; problems that are more common or carry high risk to the patient call for immediate corrective action. One common example of this kind of error is the use of unsuitable specimens, such as blood samples that are hemolyzed, clotted, of insufficient volume, or feature an inadequate ratio of blood to additive. Analyzing samples like these leads to unreliable test results. Another issue, less frequent but with higher risk to the patient, is identification error. Its rate of incidence is currently at less than one percent, but with the possibility of harmful consequences it carries, laboratories worldwide should adopt a zero tolerance attitude toward this kind of error.
The search for answers
There is ample evidence (9) of the impact that preanalytical phase errors have on patient safety. In fact, laboratory and radiologic issues have been found to account for almost half of all diagnostic errors (4). Though the impact to the patient can vary widely – from physical discomfort to potentially permanent disability or fatality – these problems can be minimized by the implementation of standardized, patient-centered, evidence-based policies and procedures. But this is no easy task – other studies (10) have shown that even if standard procedures are in place, adequate compliance is extremely difficult to ensure. Education improves compliance numbers, but even that only works in the short term; for full effectiveness, compliance education needs to be continuously offered and updated.
The problem with making evidence-based safety decisions is that it’s almost impossible for a single laboratory to collect the necessary evidence for every procedure. Preanalytical phase quality is a “hot topic” in laboratory medicine at the moment; the number of published studies has dramatically increased over the last few years. This means that many of the questions labs are asking have been answered in the literature already – but researchers must not only search for that evidence (which is not always easy to find), but also critically assess its suitability to their testing environment and specific clinical context. And for those questions that lack an evidence base, labs must research and prepare their own – because without it, making responsible decisions about routine work becomes an impossible challenge. Without research into the effect of hemolysis, for example, how can we know whether or not a hemolyzed sample is acceptable for analysis? Without evidence of the effect of delayed transport, how can we know whether a delayed sample is acceptable? It’s obvious that evidence-based decisions are needed to ensure that patients get appropriate, timely and reliable tests.
Lack of compliance and clarity
Existing guidelines aren’t always appropriately followed in the laboratory. There are several reasons behind this: if procedures have standards or recommendations at all, they’re often outdated, not universally applicable, not evidence-based, or lacking in vital details. For example, the Clinical Laboratory Standards Institute (CLSI) guidelines for venous blood collection state that laboratory staff should ensure that patients have been properly prepared for testing, but don’t explain what this preparation should be. As this is a key step in the blood collection procedure, proper patient preparation is necessary for reliable and accurate test results (see “No Blood Sample is Better than a Bad Blood Sample”). CLSI standards aren’t free, either, and many labs in developing countries aren't able to purchase them.
No Blood Sample is Better than a Bad Blood Sample
According to the WG-PA of the EFLM, existing guidelines for phlebotomy need revision (11). Standardization would allow harmonized reporting of scientific data in the field of laboratory diagnostics and revised guidelines should include:
- The exact definition of requirements for patient preparation for laboratory testing. Blood for all blood tests should be drawn preferably in the morning from 7 to 9 am. Fasting should last for 12 hours during which water consumption is permitted. Alcohol should be avoided for 24 hours before blood sampling. In the morning before blood sampling, patients should refrain from cigarette smoking and caffeine-containing drinks.
- Professional associations (IFCC, EFLM and others) should support harmonization efforts by disseminating standardized recommendations for fasting.
- Laboratories worldwide should implement standardized procedures for blood sampling and patient preparation.
- Laboratories should have policies for sample acceptance criteria related to fasting samples. Blood samples for routine testing should not be taken if a patient has not been appropriately prepared for sample collection. ‘No sample is better than a bad sample’ should always be the leading principle.
- Laboratory professionals are responsible for disseminating information about fasting requirements to patients as well as to clinicians and general practitioners who are the preferred source of patient information.
Recent work by the group (12) has also recommended the standardization of color coding for blood collection tube closures and labels to reduce the risk of preanalytical errors. They propose the following roadmap:
- All stakeholders, including all manufacturers working in the field, should be invited to join a dialog to establish a universally acceptable color coding standard for blood collection tube closures;
- Standard writing bodies (ISO, CLSI) should add the color coding standard agreed onto the existing recommendations;
- Manufacturers should implement the agreed color coding standard.
Guidelines represent the best possible practice at the time of their creation, and unless they are freely accessible, they won’t see widespread adoption. This problem is only emphasized when these practices are difficult to implement, because humans are often resistant to change. We don’t always like having to replace our well-known laboratory routines with new ones; changes of that magnitude require a lot of continuous effort, education, assessment, and monitoring. The only way to make real headway is to get all of the stakeholders involved. International professional associations can take the lead in defining best practices, whereas national ones can act as a link between overseers and individual members of their societies. National organizations can also promote the importance of standards and recommendations and encourage their implementation – in fact, a great way to begin is by establishing a national working group for preanalytical phase, which can raise awareness, promote research, and provide education. Of course, individual laboratories also carry responsibility, in their case for adopting recommendations, implementing them in routine practice, and assuring compliance. At all levels, though, education and continual improvement are the keys to success.
Small steps towards the dream
The WG-PA is quite a young group, but we’ve already had some encouraging results (see “What We’ve Found So Far”). We’ve organized three international meetings where we hosted world experts, presented new research results, and offered practical tips for dealing with preanalytical phase issues – including analyzing workflows and bottlenecks, assessing impact of biological variability, managing sample hemolysis and lipemia, dealing with patient identification errors, and tips for successful phlebotomy. A third conference has just taken place where 19 national societies in Europe were invited to present their activities to almost 600 attendees. Forums like these give organizations a voice for interactive discussion so that different views and experiences can be shared.
We’ve also conducted two large surveys – one to assess the level of training provided to personnel performing venous blood sampling, and another to evaluate the level of compliance with CLSI phlebotomy standards. We opted to look at blood sampling because it’s available worldwide, it’s the most common invasive procedure in healthcare, and it’s also the most common source of preanalytical errors – which often go unrecognized. These errors can include things like unnecessary delays, incorrect test results, or even harm to the patient or phlebotomist. We found that only a quarter of European countries have national guidelines for phlebotomy, and that it’s performed by both medical and non-medical personnel with a wide range of background education – meaning that not all patients receive the same level of care (8), especially when overall guideline compliance levels are low and critical steps like patient identification are often omitted (10).
What We've Found So Far
| Report Title | Available Online | Key Findings |
| Compliance of blood sampling procedures with the CLSI H3-A6 guidelines: An observational study by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PRE) | http://tp.txp.to/0515/report1 | • Overall compliance with CLSI guidelines is unacceptably low • Issues with a high combination of probability and potential risk of harm include patient identification and test tube labeling • Administrative staff often fail to adhere to patient identification procedures • Physicians often fail to adhere to test tube labeling policy |
| Survey of national guidelines, education and training on phlebotomy in 28 European countries: an original report by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PA) | http://tp.txp.to/0515/report2 | • The quality, compliance and critical steps in current phlebotomy need to be assessed • Existing CLSI guidelines should be adapted and used locally in areas that do not have their own guidelines • National EFLM societies must develop basic training programs and continuously educate phlebotomy staff |
| Preanalytical quality improvement. In pursuit of harmony, on behalf of European Federation for Clinical Chemistry and Laboratory Medicine (EFLM) Working group for Preanalytical Phase (WG-PRE) | http://tp.txp.to/0515/report3 | • The vast majority of laboratory errors occur in the preanalytical phase • Matters of concern include unnecessary testing, prevention of needlestick injuries, harmonization of phlebotomy practices, and quality assurance standards |
| Preanalytical quality improvement: in quality we trust | http://tp.txp.to/0515/report4 | • A summary of the potential for error in laboratory diagnostics • Matters of concern include quality indicators for the preanalytical phase, phlebotomy practices (especially in pediatric samples or for blood gas analysis), urinalysis practices, and auditing the preanalytical phase |
| Preanalytical quality improvement: from dream to reality | http://tp.txp.to/0515/report5 | • There is an inherent possibility of error in the “brain to brain cycle” of lab testing • Preanalytical errors account for 60 to 70 percent of all such problems • Though most are intercepted, nearly one-fifth of those cases result in inappropriate clinical decisions and unjustifiable increases in cost • Standardization and monitoring of preanalytical variables is vital |
| Colour coding for blood collection tube closures – a call for harmonisation | http://tp.txp.to/0515/report6 | • Blood collection tubes are identified both by label and by closure color • Tube closure colors have not been standardized between manufacturers, so labs risk error when switching brands • To reduce the risk of error and improve patient safety, tube closure and label colors should be harmonized worldwide |
| Standardization of collection requirements for fasting samples: for the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) | http://tp.txp.to/0515/report7 | • Standardized protocols for patient preparation for laboratory testing are currently lacking • Great heterogeneity exists in the definitions of “fasting” currently being used among healthcare workers and in the literature – and different types of fasting result in metabolic and hormonal differences in blood samples • Patient preparation for fasting tests must be standardized to avoid variation in results |
As a result of our work, we’ve published several recommendations, including one outlining the requirements for fasting bloodwork (11) and another calling for harmonization of color coding systems for blood collection tube closures (12) (see “No Blood Sample is Better than a Bad Blood Sample”). But this is only the beginning. In the future, we hope to provide many more guidance documents to assist our colleagues in their efforts to standardize and harmonize preanalytical phase policies and practices. Awareness of preanalytical issues is rising, and so is the interest and commitment of laboratory professionals to upholding international standards in their work. It’s not something we can achieve overnight, of course, but even small steps forward indicate that preanalytical quality management is not a dream, but a reality for the very near future.
Ana-Maria Šimundić is a specialist in laboratory medicine at the Clinical Institute of Chemistry of the University Hospital Center "Sestre milosrdnice” in Zagreb, Croatia; President of the Croatian Society for Medical Biochemistry and Laboratory Medicine; Biochemia Medica Editor-in-Chief; Executive Board Secretary of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM); and Chair of the EFLM working group preanalytical phase.
- J James, “A new, evidence-based estimate of patient harms associated with hospital care,” J Patient Safety, 9, 122–128 (2013). PMID: 23860193.
- WHO, “10 facts on patient safety,” bit.ly/1H40OtS. Accessed April 8, 2015.
- European Commission, “Patient safety,” bit.ly/TEIySc. Accessed April 8, 2015.
- GD Schiff et al., “Diagnostic Error in Medicine,” Arch Intern Med, 169, 1881–1887 (2009).
- AM Simundic, G Lippi, “Preanalytical phase – a continuous challenge for laboratory professionals,” Biochem Med (Zagreb), 22, 145–149 (2012). PMID: 22838180.
- M Plebani, P Carraro, “Mistakes in a stat laboratory: types and frequency,” Clin Chem, 43, 1348–1351 (1997). PMID: 9267312.
- P Carraro, M Plebani, “Errors in a stat laboratory: types and frequencies 10 years later,” Clin Chem, 53, 1338–1342 (2007). PMID: 17525103.
- AM Simundic et al., “Survey of national guidelines, education and training on phlebotomy in 28 European countries: an original report by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PA),” Clin Chem Lab Med, 51, 1585–1593 (2013). PMID: 23729577.
- G Lippi et al., “Overview on patient safety in healthcare and laboratory diagnostics”, Biochem Med (Zagreb), 20, 131–143 (2010).
- AM Simundic et al., “Compliance of blood sampling procedures with the CLSI H3-A6 guidelines: An observational study by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) working group for the preanalytical phase (WG-PRE)”, Clin Chem Lab Med (2014). PMID: 25536667.
- AM Simundic et al., “Standardization of collection requirements for fasting samples: for the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM),” Clin Chim Acta, 432, 33–37 (2014). PMID: 24269503.
- AM Simundic et al., “Colour coding for blood collection tube closures – a call for harmonization,” Clin Chem Lab Med, 53, 371–376 (2014). PMID: 25324449.
Ana-Maria is President of the Croatian Society of Medical Biochemistry and Laboratory Medicine, and serves as EFLM Executive Board Secretary and Chair of their working group for Preanalytical Phase (WG-PA). She is currently serving as Head of the Clinical unit for Medical Biochemistry and Analytical Toxicology at Sestre milosrdnice University Hospital center. She is an assessor for ISO 15189 Accreditation and Conformity Assessment. Since 2011, she has served as the Executive Board Secretary of the EFLM.
Ana-Maria has published several book chapters and over 80 publications, and has amassed numerous awards since 2000, including Best Young Scientist and Best Research by the Croatian Society of Medical Biochemistry and Laboratory Medicine, as well as the Per Hyltoft Petersen award by the Slovak Society of Laboratory Medicine. She has also been awarded honorary membership of the Hungarian Society for Laboratory Medicine.