A Viral Vision
Meet the new miniature biosensor that aims to enhance HIV diagnostics in the field
Over 35 million people have died of HIV since the epidemic’s peak in the 1980s (1). Today, the disease still affects tens of millions of patients per year, with sub-Saharan Africa – where one in 25 adults lives with the virus – being the hardest hit. As the battle against HIV rages on, new vaccines and treatments are being proposed (2) – but the field of diagnostics has an important role to play. Investigators from the Spanish National Research Council (CSIC) have developed a tiny but sensitive chip-based biosensor to detect HIV-1 (3).
To learn more about the nanosensor and how it helps serve LMICs, we spoke with Priscila Kosaka, a researcher at the CSIC’s Bionanomechanics Lab.
Why did you focus on HIV detection?
One of the Bionanomechanics Lab’s research goals is to develop ultra-sensitive tools for the early detection of cancer and other fatal diseases – HIV fits into that latter category.
Our HIV sensor’s story began some years ago, when we focused on detecting low-abundance biomarkers in the bloodstream. We used cancer biomarkers – carcinoembryonic antigen (CEA) and prostate-specific antigen (PSA) – as our model, spiked into undiluted bovine serum (4). The concentrations we analyzed were at least three orders of magnitude lower than the cutoffs for clinical monitoring, something we hope will be useful as new, low-concentration biomarkers are increasingly discovered by emerging proteomic tools.
In the meantime, we wanted to challenge our nanosensor in human serum – partly to spot problems related to nonspecific interactions and partly to ensure that the device would detect proteins at ultra-low concentrations in human blood. That’s when the p24 capsid protein came to our attention. It’s an intriguing biomarker because it’s only found in the blood of HIV-1 infected people – so we decided to turn our sights toward HIV detection. It wasn’t just good for our research, though; prompt identification of HIV-positive individuals during the highly infectious acute or early stage has implications for both patient management and public health interventions.
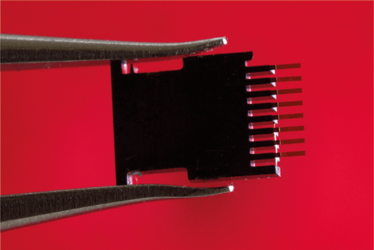
The microcantilever array used for ultra-sensitive HIV detection. Credit: Joan Costa/CSIC Communication
What are the current challenges in HIV diagnosis?
Identification of individuals in the earliest phases of infection remains a difficult task thanks to the demands of repeat HIV testing and the detection limits of current technologies.
Nucleic acid testing (NAT) is the most accurate and reliable screening platform for HIV. It’s theoretically capable of detecting HIV about two weeks after transmission – but the virus’ genetic diversity can yield false or discordant results. Moreover, most of the assays used are complex, technically demanding or inappropriate for non-specialist diagnostic laboratories.
Diagnostic platforms like ELISA are in routine use for HIV antibody detection, and the newer assays can spot both antibodies and the p24 antigen. This approach has further shortened the “window period” between infection and detection. p24 immunoassays are simpler and cheaper than NAT and have potential in low-resource settings, but their sensitivity must be improved if we’re to use them to detect early-stage infection.
How does your device work?
It’s an array of eight silicon microcantilevers that we use as biosensors. They can transform a biological signal into one we can measure. We’ve recently discovered that the microcantilevers are also good optical cavities; their two opposite surfaces work as semitransparent mirrors that trap light and boost the scattering of metallic nanoparticles. We can use that property to perform a sandwich immunoassay, functionalizing the microcantilevers with capture antibodies and dipping them into serum so that the p24 antigens can bind. Then, we incubate them with gold nanoparticles bound to detection antibodies, rinse, and measure.
Ultimately, we have two detection methods: mass and plasmonic labeling. The microcantilevers act as mechanical resonators that allow us to measure the mass of the captured nanoparticles, and they also work as optical cavities to allow localized surface plasmon resonance – crucial to achieving the ultrasensitivity we need to detect p24 in human serum.
Why is the device viable in regions with limited resources?
The microcantilever arrays and optical cavities are fabricated en masse by well-established semiconductor technology. Similarly, optical components, such as lasers and photodiodes, can be acquired at very low cost. Even microscopy is less of a challenge than ever; smartphones can now be transformed into powerful, economical optical microscopes to be used in field settings. Our nanosensor has the potential to become a cheap and user-friendly technology suitable for resource-limited settings – hopefully, in the very near future. We are working hard to turn that dream into reality!
At the moment, the cost of our sensor is still relatively high. For example, the microcantilever array costs around €20, and each individual analysis can reach €100. But when we begin large-scale production, I predict that the price tag will decrease dramatically – I can envision fabrication costs as low as €1 or less. And when that happens, I’m looking forward to a rollout in the places that could benefit most.
With just a simple two-step process, laboratory medicine professionals can have a result in under five hours, and deliver patient follow-up on the same day. It’s our goal that people with HIV will be able to start treatment as early as possible, suppressing viral replication and allowing them to keep their immune systems undamaged – and have a longer, healthier life. It will also substantially reduce the risk of transmission of the virus to uninfected people.
We don’t want to stop at HIV. We’re also currently working on projects for early cancer detection, and we’re interested in a number of other health issues – cardiac disease, Zika, Ebola, and more. We hope that, one day, we may have simple, low-cost point-of-care devices for all sorts of applications – and distribute them to all of the hospitals, clinics and healthcare professionals who need them most.
- World Health Organization, “Global health observatory (GHO) data HIV/AIDS”, (2017). Available at: bit.ly/1vM5gaL. Accessed March 21, 2017.
- T Fouts, “Finding HIV’s Achilles’ heel”, 2, 26–30 (2016). Available at: bit.ly/2nNAC1u. Accessed March 21, 2017.
- PM Kosaka et al., “Ultrasensitive detection of HIV-1 p24 antigen by a hybrid nanomechanical-optoplasmonic platform with potential for detecting HIV-1 at first week after infection”, PLoS One, 12, e0171899 (2017). PMID: 28199410.
- PM Kosaka et al., “Detection of cancer biomarkers in serum using a hybrid mechanical and optoplasmonic nanosensor”, Nat Nanotechnol, 9, 1047–1053 (2014). PMID: 25362477.