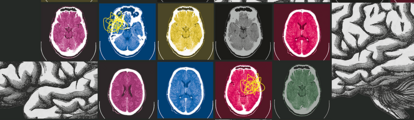
What’s in a Brain?
A guide to identifying common adult intracranial neoplasms for anatomic pathologists
At a Glance
- Intracranial neoplasms present a diagnostic challenge, but a few simple algorithms can assist in the process
- The most common intracranial tumors in adults are metastases; only consider primary CNS neoplasms after ruling out this possibility
- Meningiomas are the most common primary intracranial neoplasms of the central nervous system and its coverings and are more common than primary glial tumors
- When facing a glial tumor, distinguishing between astrocytoma, oligodendroglioma, and ependymoma can have significant effects on prognosis and treatment decision-making
As diagnostic experts, pathologists are routinely faced with challenging tissue samples. But, for many, none are so intimidating as those that originate from the brain. Although there are many areas of the brain that are particularly eloquent, there is no true way to resect brain tissue without impact to the patient. With so much at stake, it can be hard to confidently render a diagnosis – and, indeed, many consider central nervous system (CNS) tumors among the most intimidating, particularly in terms of intraoperative assessment. In reality, these tumors are no more intimidating than any other, and a few handy tips, tricks, and algorithms can help any pathologist tackle common intracranial neoplasms.
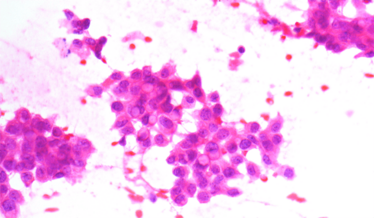
Carcinoma smear.
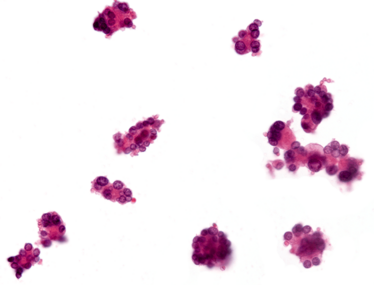
Cohesive clusters in metastatic cancer.
Many pathologists will be familiar with the concept of integrated diagnosis. Hematopathologists, for instance, have been working in an integrated fashion for well over a decade. But it’s a far more recent phenomenon in soft tissue pathology, where morphology and histology have long been the definitive gold standard for interpretation. Many specialties within pathology now use a synthesis of histology and ancillary testing – including, in many cases, molecular testing. Integration is key; no one type of information dominates over any other. One of my favorite examples lies in the comparison of angiomatoid fibrous histiocytoma and clear cell sarcoma. The two have exactly the same translocation (EWSR1/ATF1), but angiomatoid fibrous histiocytoma has a typically benign prognosis, whereas clear cell sarcoma kills about half the patients who receive the diagnosis. So the same monogenetic event, with a different site and a different histology, yields a wildly different outcome.
The same applies to brain tumors. A nice example of this pathology is ganglioglioma – a WHO grade 1 tumor frequently associated with the classic BRAF V600E mutation. These tumors typically have a benign course, with many patients enjoying a normal lifespan following curative surgery. Nonetheless, the same mutation appears in epithelioid glioblastoma, a WHO grade 4 tumor with an abysmal prognosis. You can’t just put tumor tissue in a blender, fish for genetic changes, and assume that this will yield a diagnosis. You have to examine the histology, decide what testing would contribute to the diagnosis, and interpret the test results in the context of morphology. No one test alone is enough; it’s the combination that holds the diagnostic key.
The intraoperative assessment
Many anatomic pathologists are responsible for intraoperative assessments for neuropathologic specimens (some consider these to be the most challenging frozen sections they face). Depending on the surgical procedure and clinical situation, a frozen may play a vital role… or may be deferred with no consequence for the patient whatsoever. The key is to know which situation you’re in.
When dealing with a biopsy – particularly a stereotactic biopsy – pathology is absolutely essential. One of the classic indications for a stereotactic biopsy would be distinguishing between a high-grade glioma and a lymphoma – this is a high-stakes scenario in the brain, as the safely resectable enhancing tumor will be removed, whereas a diagnosis of lymphoma does not indicate surgical intervention, giving us a classic “cut it out or not” scenario. If, however, the surgeon intends to resect a space-occupying mass, the stakes are somewhat lower. The surgeon is already planning to cut out as much grossly abnormal tissue as possible and is unlikely to change that plan based on the pathology.
Tip
If the procedure is a biopsy, the pathologist must let the surgeon know if the tissue is diagnostic, as well as if the pathology does not indicate further surgical intervention (for instance, in the case of lymphoma or infection).
In the background, the pathologist has to keep in mind the neuroanatomy of the biopsy site. By way of example, the cerebellum has a granular neuron layer which, when examining a smear or frozen section, may (falsely) appear to be hypercellular and set in a fibrillary background; granular neurons can resemble lymphocytes on a smear preparation. It’s always a good idea to know what part of the brain
you’re examining.
Some pathologists prefer to read the radiology report prior to assessing the histology; others – like me – prefer to review it after seeing the histology to confirm (or contest) their independent assessment. An absence of concordance between the radiology and histology prompts closer examination and re-evaluation of the tissue.
Common intracranial pathologies
What’s the most common intracranial lesion in adults? If you answered with any primary CNS lesion, think again. The most common intracranial lesion in adults is a metastasis – and we’re seeing increasing numbers of them as we get better at treating stage IV cancer and identifying situations in which resection carries symptomatic (or even survival!) benefit. There are some diseases – like breast or lung cancer – in which the surgical and oncology teams can achieve systemic control that, as long as the brain metastases are resected and treated with postoperative radiosurgery, can achieve near-curative outcomes in metastatic cancer.
Tip
When examining tumor samples from the brain, remember to consider the possibility of metastasis before diagnosing a primary CNS neoplasm.
I have noticed that, sometimes, my residents (who would have no trouble recognizing cancers occurring in their native organs) neglect to consider metastatic pathologies when examining tumor samples from the brain. Breast cancer and melanoma are good examples of lesions that can have abundant eosinophilic cytoplasm. Frozen sections are not always perfect representations of the morphology of a tumor, so if you see a homogenous pink “thing” with atypical nuclei, you may be tempted to diagnose a primary glial neoplasm. If you are comfortable with neuropathological smear samples, it’s advisable to look for convincing evidence of glial differentiation; for instance, cytoplasm that is drawn out into long, slender, hair-like processes.
It’s also not uncommon for a brain metastasis to be detected before the primary. Melanoma and small cell carcinomas are two common examples. In the case of melanoma, the primary may go unnoticed, especially if it doesn’t arise on visible skin, or the cutaneous melanoma may have regressed. Lung cancer is another disease family in which brain tumors may be diagnosed either first or simultaneously with the disease in the lung. Interestingly, in cases where the brain metastasis is symptomatic, the CNS material will be used to assess the molecular profile of the tumor – especially if the primary tumor can’t be resected.
For every sample I encounter in the frozen section room, I ask myself:
- Is this normal or abnormal? That is, does any native brain tissue look like the sample? If not, then I proceed down the abnormal branch.
- Is it neoplastic or reactive/inflammatory? We’re often so quick to jump to tumors that we forget that diseases like progressive multifocal leukoencephalopathy, organizing infarcts, or demyelinating disease can all have features reminiscent of a tumor – but they are not neoplastic. That’s a key step; never skip that question.
- Is this of the CNS and/or its coverings? Including the meninges, is this a brain or meningothelial tumor? If the answer is no, it’s time to consider metastasis.
Tip
To diagnose a primary glial neoplasm, look for evidence so clear that a resident would unequivocally call a glial process; otherwise, you may be seeing artifacts of the smear process.
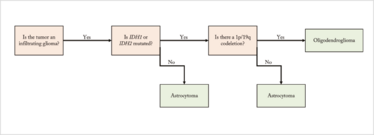
Figure 1. Diagnostic algorithm for primary glial tumors.
In fact, glioma isn’t even the second most common intracranial pathology in adults. That honor falls to meningioma – which is an easier diagnostic entity. Not only can the surgeon tell you whether the tumor is attached to or arising from the dura, but the radiology is fairly typical; meningiomas are extra-axial (outside the brain parenchyma) and attached to the dura mater. One of the unfortunate challenges of meningioma smears is that meningothelial cells have abundant “diaphanous” cytoplasm (usually semi-translucent and white or pink) and form tight junctions with their neighbors with innumerable interdigitating filaments – so when these are smeared out, they can mimic glial processes.
But there’s one key way to spot a meningioma: the whorls. Almost every meningioma features true whorl formation somewhere. One nice trick is to perform a touch prep instead of a smear. You may see less overall, but you’ll be able to identify the whorls, as well as some other common features such as psammoma bodies. Meningiomas are more common than gliomas!
Tip
A combination of cytologic preparations and frozen sections, along with radiology and history, can help you diagnose meningioma.
The third most common intracranial neoplasm in adults is a primary glial tumor – specifically glioblastoma. This WHO grade 4 tumor is by far the most common glioma in adults. When you perform your smear, you may see eosinophilic glial processes; you may see a fair degree of pleomorphism; you may see mitotic activity. The defining characteristic, though, is the presence of microvascular proliferation and/or necrosis.
Must you always make a diagnosis of glioblastoma in the intraoperative arena? Not necessarily. Identifying a glial tumor should be enough to guide the surgeon, who will integrate your intraoperative assessment with the radiology and the gross appearance under the dissecting microscope. Because these tumors are so infiltrative, it’s impossible to truly resect them in their entirety. The surgeon’s role in the treatment of glioblastoma is to maximally remove the enhancing component of the tumor – and if they are successful, it’s considered a gross total resection. Glioblastoma shares many characteristics with other glial tumors, such as oligodendrogliomas, and differentiating between the diagnoses often requires not just histological, but also molecular, investigation.
Tip
Diagnosing a glial tumor intraoperatively is often enough to provide adequate surgical guidance; identifying the particular type and grade of tumor can wait for molecular testing.
The glial tumors
There are three main types of glial tumor seen in adults: astrocytoma, oligodendroglioma, and ependymoma (in order of incidence).
Ependymomas are unusual in that they are non-infiltrating – which means that they can be cured by surgical resection. The classic histologic feature is the perivascular pseudorosettes, and the ancillary immunohistochemical test is epithelial membrane antigen (EMA). If you see perinuclear, dot-like positivity on EMA testing, you can confidently diagnose ependymoma.
The world of infiltrating glioma is more complicated. Essentially, you have two choices: astrocytoma or oligodendroglioma. A helpful diagnostic algorithm (see Figure 1) can allow you to distinguish between the two.
Tip
Oligoastrocytoma is an unhelpful diagnosis for neuro-oncologists – and many neuropathologists don’t believe it is a true molecular entity.
The most important molecular alteration in infiltrating gliomas is mutation in one of the isocitrate dehydrogenase genes. The most common, which accounts for 90 percent of IDH mutations in gliomas, is the IDH1 R132H mutation. Fortunately, we have mutation-specific immunohistochemical testing to identify this alteration, which we perform on all infiltrating gliomas.
If that test is negative and either the morphology looks like oligodendroglioma or the patient is under 65, we proceed to secondary IDH testing. Here in Montreal, we use three different tests: immunohistochemistry, Sanger sequencing, and SNaPshot sequencing. SNaPshot tests for the most common mutations in both IDH1 and IDH2, and it’s quite satisfying how many times the tumor resembles an oligodendroglioma, but the immunohistochemistry is negative – and then we find either an unusual IDH1 or, more commonly, an IDH2 mutation.
Tip
If a tumor resembles an oligodendroglioma, but immunohistochemistry is negative, test for less common mutations in both IDH1 and IDH2 before making a final diagnosis.
If neither IDH gene is mutated, you can safely diagnose astrocytoma. If IDH1 or IDH2 is mutated, the next question is the status of 1p/19q co-deletion, a large cytogenetic alteration required to diagnose oligodendroglioma. Many centers test this by fluorescence in situ hybridization, but some use loss of heterozygosity studies or next generation sequencing for copy number alteration. Regardless of your testing platform, if the tumor shows IDH mutations and 1p/19q co-deletion, you can unequivocally diagnose oligodendroglioma.
Grading a glioma
Astrocytomas come in three grades: WHO grade 2, 3, or 4. As soon as you have hypercellularity and nuclear atypia, you know an infiltrating astrocytoma is at least grade 2. The addition of mitotic activity should prompt an upgrade to anaplastic (grade 3) astrocytoma.
Tip
Evaluation of mitotic activity can be somewhat subjective, even one mitotic figure on a needle biopsy or in a small amount of tissue is enough to increase the tumor grade.
If, on top of those characteristics, you also see palisading necrosis and/or microvascular proliferation, you can diagnose a glioblastoma (grade 4) tumor. A nice trick for spotting microvascular proliferation is so-called “endothelial duplication.” In normal brain tissue, the endothelial cells of the blood vessels are flat and elongated, with eccentric nuclei, and they almost never line up. In microvascular proliferation, you can often find two endothelial cells sitting on top of one another other on the same side of the lumen – endothelial duplication. If you see that, you are likely dealing with microvascular proliferation, indicating a diagnosis of glioblastoma multiforme.
Unlike astrocytomas, oligodendrogliomas only come in two grades: WHO grade 2 or 3. If you see only oligodendroglial proliferation, you have a grade 2 tumor; if you see a microvascular proliferation or necrosis, you should upgrade the diagnosis to anaplastic (grade 3) oligodendroglioma. Many neuropathologists also factor in mitotic activity, although this is
less reproducible.
Ultimately, why is the correct diagnosis and grade so vital? Because the survival difference between an astrocytoma and an oligodendroglioma can be well over a decade. An IDH-mutated tumor, even a glioblastoma, has a much better prognosis than an IDH wild-type tumor. Furthermore, strong evidence suggests that patients with oligodendrogliomas benefit from postoperative chemo and radiotherapy.
Jason Karamchandani is Associate Professor in McGill University’s Department of Pathology, and a practicing neuropathologist at The Montreal Neurological Institute and Hospital, Montreal, Canada.
Jason Karamchandani is Associate Professor in McGill University’s Department of Pathology, and a neuropathologist at The Montreal Neurological Institute and Hospital, Montreal, Canada